
Introduction
- Determination of Sodium (Na⁺) and potassium (K⁺) ions is critical electrolytes in the human body, involved in various physiological processes like fluid balance, nerve transmission, and muscle contraction.
- In clinical biochemistry labs, measuring Na⁺ and K⁺ levels in biological fluids such as blood, plasma, and urine is crucial for diagnosing electrolyte imbalances, kidney disorders, and heart conditions.
- Several methods are used to determine Na⁺ and K⁺ concentrations in biochemistry labs, including Flame Photometry and Ion-Selective Electrode (ISE) Technology.
Flame Photometry Method
Flame photometry (also known as flame emission spectroscopy) is commonly used in biochemistry labs to measure the concentration of alkali and alkaline earth metals, such as Na⁺ and K⁺, in biological fluids.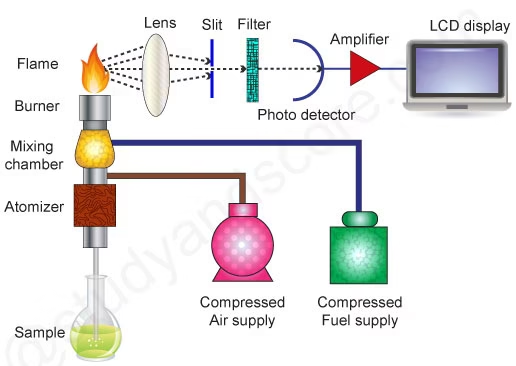
Principle:
- When a solution containing sodium or potassium ions is introduced into a flame, the flame’s heat excites the ions.
- As the ions return to their ground state, they emit light at characteristic wavelengths.
- Sodium emits light at 589 nm (yellow) and potassium at 766 nm (violet).
- The intensity of the emitted light is directly proportional to the concentration of the ions in the sample.
Procedure:
- Sample Preparation:
- The sample (e.g., serum, plasma, or urine) is diluted with deionised water or an appropriate diluent.
- Nebulization:
- The liquid sample is introduced into the flame using a nebuliser, which converts the sample into a fine aerosol.
- Flame Atomization:
- The sample aerosol is atomized in the flame, where Na⁺ and K⁺ ions are excited and emit light of characteristic wavelengths.
- Light Detection:
- A monochromator selects the wavelength specific to Na⁺ or K⁺, and a photomultiplier detects the emitted light.
- Concentration Calculation:
- The intensity of the emitted light is measured, and the concentration of Na⁺ and K⁺ is determined by comparing the intensity to that of standard solutions of known concentrations.
Advantages:
- Quick and simple.
- Cost-effective.
- Suitable for routine analysis of Na⁺ and K⁺ in clinical samples.
Disadvantages:
- Limited sensitivity for very low concentrations.
- Potential interference from other ions or contaminants.
Ion-Selective Electrode (ISE) Method
Due to its specificity and speed, the Ion-Selective Electrode (ISE) method is the most commonly used technique for measuring sodium and potassium in modern clinical laboratories.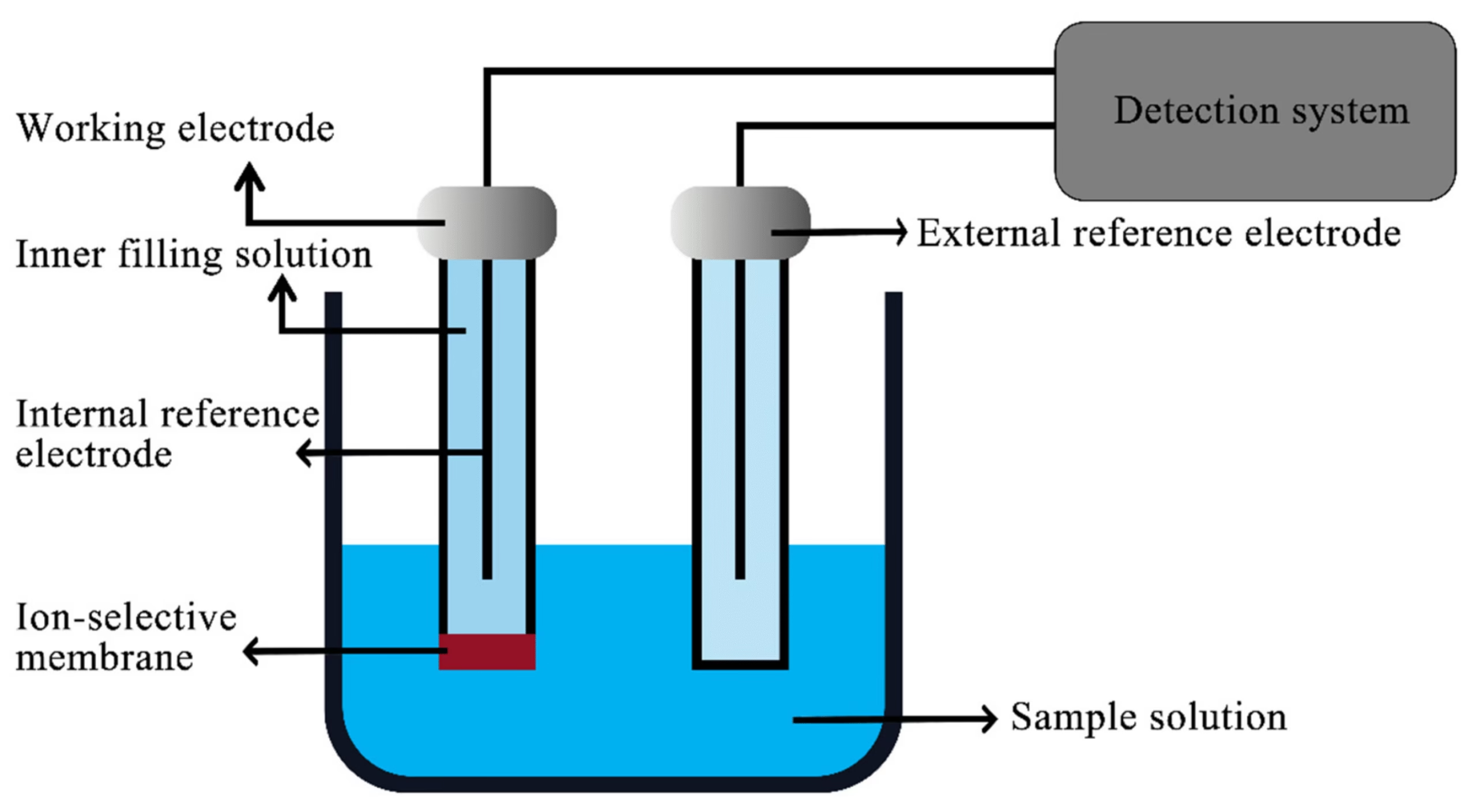
Principle:
- ISEs use a membrane that selectively binds to specific ions (Na⁺ or K⁺) in the sample.
- When the sample is in contact with the ion-selective membrane, a potential difference (voltage) is generated due to the movement of ions.
- This potential is measured relative to a reference electrode, and the concentration of the ion is determined based on the Nernst equation.
Procedure:
- Sample Preparation:
- The biological sample (serum, plasma, urine) is directly or slightly diluted.
- Electrode Setup:
- Two ion-selective electrodes, one specific to Na⁺ and the other to K⁺, are immersed in the sample.
- Potential Measurement:
- The electrodes measure the potential difference between the ion-selective and reference electrodes. The magnitude of the potential difference is directly proportional to the ion concentration in the sample.
- Calibration:
- The ISE system is calibrated using standard solutions with known Na⁺ and K⁺ concentrations to create a reference curve.
- Concentration Determination:
- The measured potential difference is compared to the calibration curve to determine the concentration of Na⁺ and K⁺ in the sample.
Advantages:
- High specificity for Na⁺ and K⁺ ions.
- Rapid and real-time results.
- Minimal sample preparation is required.
- It is accurate and precise, especially for clinical samples.
- Non-destructive (the sample remains intact after measurement).
Disadvantages:
- Membrane fouling or degradation over time requires regular calibration.
- Sensitive to interference from other ions or substances in the sample.
Clinical Significance of Sodium (Na⁺)
Sodium (Na⁺) is the major cation in extracellular fluid and plays a key role in regulating fluid balance, nerve function, and muscle contractions. Normal serum sodium levels range from 135–145 mmol/L.
1. Hyponatremia (Low Sodium Levels):
- Definition: Serum Na⁺ level <135 mmol/L.
- Causes:
- Excessive water intake (dilutional hyponatremia).
- Heart failure, kidney disease, or liver cirrhosis: These conditions cause fluid retention, diluting sodium levels.
- Syndrome of Inappropriate Antidiuretic Hormone (SIADH): Leads to water retention, causing low sodium levels.
- Diuretic use: Certain medications cause excessive sodium loss.
- Symptoms:
- Nausea, headache, confusion, muscle weakness.
- Severe hyponatremia can lead to seizures, coma, or even death.
- Clinical Implications:
- Immediate treatment is required to prevent complications like brain swelling (cerebral edema) caused by the osmotic shift of water into cells.
2. Hypernatremia (High Sodium Levels):
- Definition: Serum Na⁺ level >145 mmol/L.
- Causes:
- Dehydration due to inadequate water intake or excessive water loss (e.g., through sweating, diarrhea).
- Diabetes insipidus: Causes excessive urine production, leading to dehydration.
- Excessive sodium intake: Either through diet or intravenous saline.
- Symptoms:
- Thirst, dry mouth, irritability, confusion, muscle twitching.
- Severe hypernatremia can cause brain cell shrinkage, leading to seizures, coma, and death.
- Clinical Implications:
- Hypernatremia often indicates a water deficit or an underlying disorder affecting water regulation. It is critical to correct this imbalance gradually to prevent complications.
Clinical Significance of Potassium (K⁺)
Potassium (K⁺) is the major intracellular cation, essential for maintaining cell membrane potential, nerve conduction, and muscle contraction (including the heart). Normal serum potassium levels range from 3.5–5.0 mmol/L.
1. Hypokalemia (Low Potassium Levels):
- Definition: Serum K⁺ level <3.5 mmol/L.
- Causes:
- Diuretic use: Potassium-wasting diuretics (e.g., furosemide) cause excessive K⁺ loss.
- Vomiting, diarrhea, or excessive sweating: Leads to potassium depletion.
- Hyperaldosteronism: Elevated aldosterone levels cause potassium loss.
- Inadequate dietary intake or malabsorption.
- Symptoms:
- Muscle weakness, fatigue, cramps, arrhythmias (irregular heartbeats), paralysis in severe cases.
- Clinical Implications:
- Severe hypokalemia can cause life-threatening cardiac arrhythmias like ventricular fibrillation or asystole. Immediate correction is necessary, often with intravenous potassium supplementation.
2. Hyperkalemia (High Potassium Levels):
- Definition: Serum K⁺ level >5.0 mmol/L.
- Causes:
- Kidney failure: Impaired kidney function leads to decreased potassium excretion.
- Medications such as potassium-sparing diuretics, ACE inhibitors, and NSAIDs.
- Hemolysis: Release of intracellular potassium into the bloodstream.
- Metabolic acidosis: As hydrogen ions move into cells, potassium shifts from inside to the blood.
- Symptoms:
- Nausea, fatigue, weakness, and, more importantly, life-threatening cardiac arrhythmias like ventricular tachycardia, fibrillation, or cardiac arrest.
- Clinical Implications:
- Hyperkalemia requires immediate medical intervention, as elevated potassium levels can cause sudden cardiac arrest. Treatments include potassium-binding agents, insulin with glucose (to drive K⁺ back into cells), and dialysis.
Diagnostic and Clinical Applications
- Assessment of Kidney Function:
- Sodium and potassium levels are commonly measured to assess kidney function. In kidney diseases, the excretion of these electrolytes can be impaired, leading to hyperkalemia and fluid retention (affecting Na⁺ levels).
- Monitoring of Fluid and Electrolyte Balance:
- Na⁺ and K⁺ levels are key indicators of fluid and electrolyte balance in conditions like dehydration, overhydration, or heart failure.
- Sodium levels are critical for assessing fluid shifts, and potassium levels are crucial for evaluating the effects on cellular functions and cardiac health.
- Cardiac Health Monitoring:
- Potassium plays a crucial role in cardiac electrophysiology. Hypokalemia or hyperkalemia can lead to severe arrhythmias and are monitored in patients with heart disease, on diuretics, or those receiving chemotherapy.
- Endocrine Disorders:
- Disorders such as Cushing’s syndrome (which increases sodium retention) or Addison’s disease (which decreases sodium and increases potassium levels) are diagnosed and monitored using Na⁺ and K⁺ measurements.
- Critical Care and Intensive Monitoring:
- In ICU patients, electrolyte monitoring is essential. Sodium and potassium imbalances are often seen in patients on mechanical ventilation, those receiving intravenous fluids, or those with shock, trauma, or sepsis.
- Medication Management:
- Certain drugs (e.g., ACE inhibitors, diuretics, corticosteroids) affect sodium and potassium levels. Monitoring these electrolytes helps prevent complications, such as hyperkalemia from potassium-sparing diuretics.