
- Electron microscopy is a powerful imaging technique that uses electron beams instead of light to achieve high-resolution images of specimens at the nanometer scale.
- This allows for detailed visualization of cell structures, molecular arrangements, and surface textures that cannot be seen with conventional light microscopy.
Types of Electron Microscopes
1. Transmission Electron Microscope (TEM)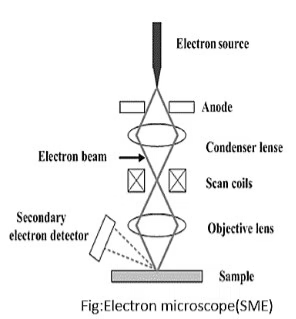
Working Principle:
- In TEM, a high-energy electron beam is produced by an electron gun, typically using a tungsten filament or a field emission gun. The beam is then condensed and focused onto the specimen by the condenser lens system.
- As the electrons pass through the specimen, they interact with the atoms and can be transmitted or scattered. The extent of scattering depends on the density and composition of the material. The objective lens then focuses the transmitted electrons to form an image on a fluorescent screen or a digital camera.
Key Features:
- Resolution: TEM can achieve resolutions better than 1 nm, enabling the observation of individual atoms in materials.
- Contrast Mechanisms: Contrast in TEM images arises from differences in electron density, the thickness of the specimen, and the atomic number of the elements present.
Typical Applications:
- Analysis of cell organelles, such as the mitochondria and endoplasmic reticulum.
- Structural analysis of viruses, proteins, and nanoscale materials.
- Characterization of crystal structures and defects in materials science.
Components:
- Electron Gun: Generates the electron beam, often using a tungsten filament heated to produce thermionic emission or a field emission source for higher brightness and coherence.
- Condenser Lenses: Focus the electron beam onto the specimen; multiple lenses can be used to achieve a small spot size.
- Specimen Stage: Holds the specimen and allows for precise movement (e.g., tilting and rotation) during imaging.
- Objective Lens: Primary lens that magnifies the image created by the transmitted electrons.
- Intermediate and Projector Lenses: Further magnify and project the image onto a screen or detector.
- Detector System: Includes photographic films, fluorescent screens, or digital cameras for image capture.
2. Scanning Electron Microscope (SEM)
Working Principle:
- SEM operates by scanning a finely focused electron beam across the surface of a specimen. The electrons interact with the atoms in the specimen, producing secondary electrons (for surface imaging), backscattered electrons (for compositional contrast), and characteristic X-rays (for elemental analysis).
- The emitted signals are collected by detectors and processed to create a high-resolution, three-dimensional image of the specimen surface.
Key Features:
- Depth of Field: SEM provides a greater field depth than optical microscopy, allowing for clear imaging of the specimen’s surface morphology.
- Three-Dimensional Imaging: By scanning the surface and compiling data, SEM can produce three-dimensional representations of the specimen.
Typical Applications:
- Surface morphology analysis of biological specimens, such as tissues, cells, and bacteria.
- Examination of materials, including fractures, coatings, and composites.
- Semiconductor and nanotechnology research for characterizing microstructures.
Components:
- Electron Gun: Generates and focuses the electron beam; often employs a tungsten filament or a field emission source.
- Condenser Lenses: Focuses the electron beam onto the sample surface.
- Scanning Coils: Direct the beam across the specimen in a raster pattern, allowing for scanning.
- Detectors: Collect emitted signals:
- Secondary Electron Detector: Most commonly used for imaging surface topography.
- Backscattered Electron Detector: Provides information about elemental composition and contrast based on atomic number.
- X-ray Detector (EDX): For elemental analysis, detects X-rays emitted during electron interactions.
Allied Techniques for Electron Microscopy
1. Cryo-Electron Microscopy (Cryo-EM)
Overview:
- Cryo-EM is a variant of TEM that enables the imaging of biological specimens in their native hydrated states by rapidly freezing them. This avoids the artifacts that may arise from chemical fixation and dehydration.
Key Advantages:
- Preserving biological samples in their near-native state allows for better structural analysis of macromolecules.
- No need for staining, which can introduce artifacts or alter structures.
Applications:
- Structural biology to study protein complexes, ribosomes, and membrane proteins.
- Visualization of cellular structures, such as membranes and cytoskeleton components.
2. Electron Tomography
Overview:
- Electron tomography is a technique to obtain specimens’ three-dimensional (3D) structures from a series of 2D images taken at different angles.
Process:
- Multiple images of the same specimen are collected at different tilt angles.
- Computational algorithms reconstruct these images into a 3D representation, allowing for detailed spatial analysis of complex structures.
Applications:
- Studying the 3D architecture of cells, organelles, and other biological structures at high resolution.
- Analysis of material properties and defects.
3. Energy-dispersive X-ray Spectroscopy (EDX/EDS)
Overview:
- EDX is an analytical technique commonly used in conjunction with SEM and TEM to determine the elemental composition of a specimen.
Process:
- When the electron beam interacts with the specimen, it excites the atoms, causing them to emit characteristic X-rays.
- The EDX detector measures the energy and intensity of the emitted X-rays, allowing for identifying elements present in the sample.
Applications:
- Elemental analysis of biological tissues, minerals, and materials.
- Characterization of nanomaterials and coatings.
4. Immunoelectron Microscopy
Overview:
- This technique combines immunolabeling with electron microscopy to visualize specific proteins or antigens in cellular contexts.
Process:
- Specimens are treated with antibodies that bind to the target antigen.
- The antibodies are conjugated to electron-dense markers, such as colloidal gold.
- The sample is imaged using TEM, revealing the location of the labeled antigens.
Applications:
- Localizing proteins within cells, such as receptors, enzymes, and structural proteins.
- Studying virus-host interactions by identifying viral proteins in infected cells.
5. Focused Ion Beam (FIB) Microscopy
Overview:
- FIB is often used with SEM for sample preparation and imaging, allowing for precise milling and cross-sectional imaging.
Process:
- A focused beam of ions (commonly gallium) is used to sputter away material from the surface of a specimen, creating a cross-section or shaping the sample for analysis.
Applications:
- Preparing thin samples for TEM by milling the surface to a suitable thickness.
- Creating patterns on materials for microfabrication and nanotechnology applications.
Specimen Preparation Techniques for Electron Microscopy
Sample preparation is crucial for obtaining high-quality images in electron microscopy. The methods vary depending on the type of specimen and the desired outcomes.
- Fixation
- Chemical Fixation: Commonly involves using glutaraldehyde to cross-link proteins and osmium tetroxide to stabilize lipids. This preserves cellular architecture but can sometimes introduce artifacts.
- Cryofixation: Involves rapid freezing of samples (often liquid nitrogen or ethane) to preserve structures close to their natural condition, preventing ice crystal formation that can damage cellular integrity.
- Dehydration
- Graded Alcohol Series: Specimens are sequentially immersed in increasing concentrations of ethanol or acetone, removing water and preventing structural collapse during embedding.
- Critical Point Drying (CPD): For SEM samples, this method transitions a liquid (usually ethanol) to gas without passing through a liquid phase, thus preventing surface tension artifacts.
- Embedding and Sectioning
- Embedding: After fixation and dehydration, samples are embedded in resin (e.g., epoxy or acrylic) and polymerized to provide structural support for ultra-thin sectioning.
- Ultramicrotomy: Sections are cut to a 50-100 nm thickness using an ultramicrotome equipped with a diamond knife. These thin sections are essential for TEM, allowing electrons to pass through the specimen.
- Staining
- Heavy Metal Staining: Common stains include uranyl acetate (which enhances contrast by binding to RNA and other nucleic acids) and lead citrate (which provides contrast for proteins). These stains increase electron density in cellular structures, enhancing visibility under the microscope.
- Coating (for SEM)
- Sputter Coating: Non-conductive specimens must be coated with a thin layer of conductive material (e.g., gold, platinum, or carbon) to prevent charging during electron beam exposure, which would distort the image.
Advantages and Limitations of Electron Microscopy
Advantages
- Exceptional Resolution: The capability of imaging structures at the atomic level (down to 0.1 nm in some cases) allows for detailed structural analysis.
- Elemental and Compositional Analysis: Techniques such as EDX provide insights into the elemental makeup of materials and biological tissues.
- Variety of Techniques: Many methods, including cryo-EM and immunoelectron microscopy, offer flexibility in addressing various scientific questions.
Limitations
- Complex Sample Preparation: Requires meticulous preparation, which can introduce artifacts or alter natural structures.
- Vacuum Environment: Most electron microscopy requires samples to be in a vacuum, preventing the imaging of living cells in real-time.
- Cost and Expertise: Electron microscopes are expensive to acquire and maintain, requiring skilled operators for both operation and sample preparation.