Introduction
- To estimate basal and maximal acid output (BAO and MAO) of gastric juice by determining free and total acidity under fasting and stimulated conditions.
-
Free Acidity:
This represents the concentration of free hydrochloric acid (HCl) that is not bound to proteins or other substances. It is the strongest acid component responsible for the low pH of gastric juice.Total Acidity:
Includes free HCl + combined HCl + other organic acids (lactic acid, fatty acids). It represents the total acid content that can be titrated with a base. - This helps evaluate gastric secretory function, identify pathological conditions, and guide clinical management.
Free and Total acidity of gastric juice refers to the concentration of hydrogen ions (H⁺) in the gastric juice, secreted by the parietal cells of the stomach lining. The acidity is essential for:
-
Protein digestion (via activation of pepsinogen to pepsin)
-
Sterilisation of ingested food
-
Regulation of gastric emptying
Properties of Normal Gastric Juice
| Property | Normal Value/Feature | |
|---|---|---|
| Volume | 20–50 mL (fasting) 2–3 L/day |
|
| Color | Colorless to pale yellow | |
| Odor | Slightly acidic or odorless | |
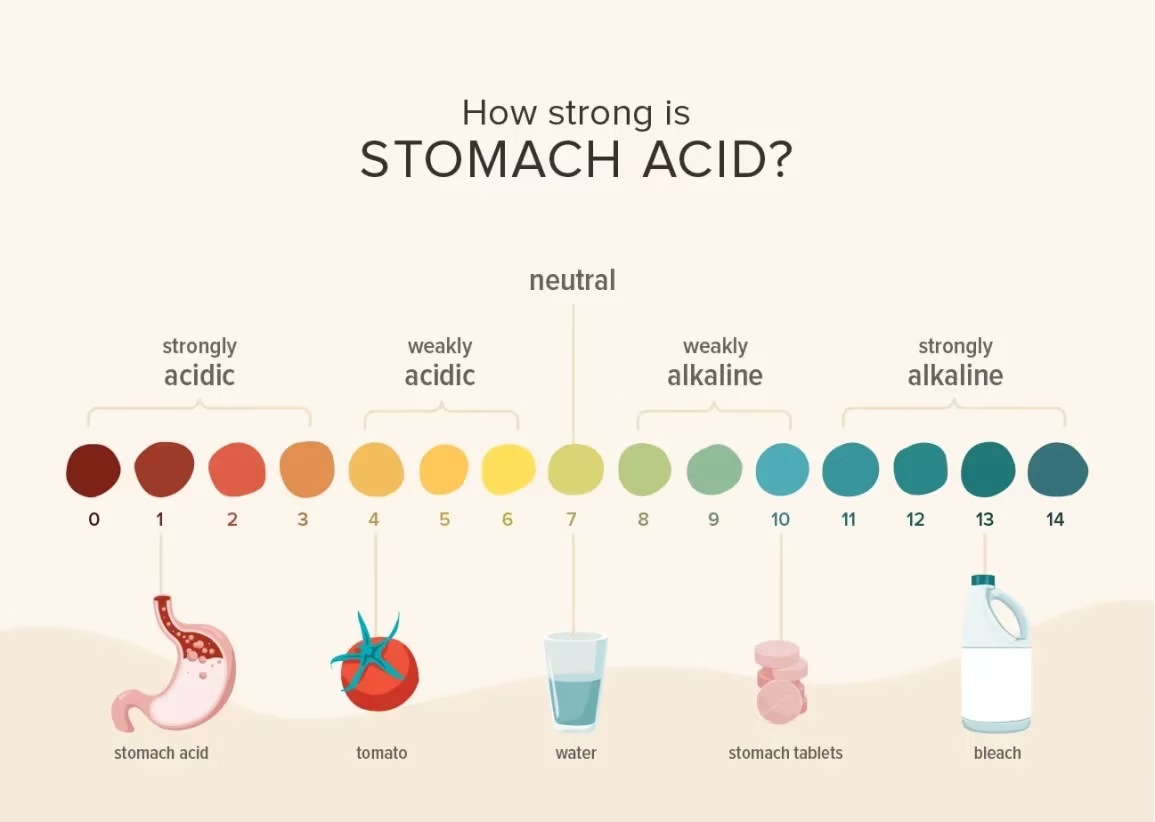
| pH | 1.6–1.8 (fasting) | |
| Free HCl | 0.4–0.5 mol/L (normal fasting) | |
| Total Acidity | 10–15 mEq/L | |
| Pepsinogen / Pepsin | Present (inactive → active pepsin in acidic pH) | |
| Mucin | Present | |
| Intrinsic Factor | Present (from parietal cells) | |
| Electrolytes | Na⁺, K⁺, Cl⁻, H⁺ (high H⁺ and Cl⁻) | |
| Enzymes | Pepsin, gastric lipase | |
| Bicarbonate | Traces (from mucosal surface, not glands) |
Physiology of Gastric Secretion
| Component | Source | Function |
|---|---|---|
| Hydrochloric acid (HCl) | Parietal cells | Lowers pH, activates pepsinogen, kills microbes |
| Pepsinogen | Chief cells | Converted to pepsin → digests proteins |
| Intrinsic factor | Parietal cells | Required for vitamin B₁₂ absorption in ileum |
| Mucus & Bicarbonate | Mucous neck cells | Protects mucosa from acid and enzymes |
| Gastric lipase | Chief cells | Hydrolyzes triglycerides (minor role) |
| Electrolytes | Gastric glands | Maintain osmolarity and pH |
| Water | Glandular secretions | Medium for enzyme activity |
Role of HCl:
-
Activates pepsinogen → pepsin
-
Maintains acidic pH (1.0–3.0)
-
Denatured dietary proteins
-
Provides bactericidal action
-
Facilitates iron and calcium absorption
Sample Collection
Pre-Test Instructions:
-
Fasting: 10–12 hours
-
No antacids, PPIs, H2 blockers 24–48 hours before the test
-
Avoid smoking, alcohol, or stimulants
Procedure:
-
Patient Position: Semi-reclining
-
Insertion of Ryle’s Tube:
-
Through nostril → esophagus → stomach
-
Confirm position by aspiration or X-ray (radio-opaque marker)
-
-
Gastric Residue Collection:
-
Aspirate residual contents to ensure baseline condition
-
pH of this is recorded (normally <2)
-
Basal and maximal acid output
1. BAO (Basal Acid Output):
-
After emptying the stomach, aspirate 2–4 samples at 15-minute intervals
-
Patient at rest, no stimulation
2. MAO (Maximal Acid Output):
-
Administer stimulant (e.g., Pentagastrin)
-
Collect 4 samples at 15-minute intervals
-
Reflects maximal parietal cell capacity
Principle of the Test
The test is based on acid-base titration:
-
Free HCl is titrated with 0.1 N NaOH using Topfer’s reagent as an indicator.
-
Remaining acids are titrated using phenolphthalein.
-
End points are color changes:
-
Topfer’s (red → yellow)
-
Phenolphthalein (colorless → pink)
-
Reagents Used
-
0.1 N NaOH (Sodium Hydroxide): standard base
-
Topfer’s Reagent (Dimethylaminoazobenzene):
-
Turns red in the presence of free HCl
-
-
Phenolphthalein: for total acidity
-
Indicates the presence of weak acids once strong acids are neutralised (pH ~8.3)
-
Procedure
-
Take 5 ml of gastric juice in a conical flask.
-
Add a drop of Topfer’s reagent.
-
The solution turns red in the presence of free HCl.
-
-
Titrate with 0.1 N NaOH until the red color disappears.
-
Note the volume (V1) → Free acidity.
-
-
Add a drop of phenolphthalein.
-
Continue titration until a permanent light pink color appears.
-
Note the additional volume (V2 – V1) → Combined acidity
-
-
Total volume (V2) gives Total Acidity.
Calculation
Acidity is expressed in mEq/L (milliequivalents per liter) using the formula:
Acidity (mEq/L) = Volume of NaOH used (in mL) × Normality × 1000 / Volume of gastric juice used (in mL)
For 5 mL sample and N/10 NaOH:
Acidity (mEq/L)=Volume of NaOH used×20
Normal Reference Values
| Parameter | Normal Range |
|---|---|
| Free acidity | 0–30 mEq/L |
| Total acidity | 10–50 mEq/L |
| BAO | 1–5 mEq/hour |
| MAO | 10–25 mEq/hour |
| BAO/MAO ratio | <0.3 (↑ in Z-E syndrome) |
Interpretation & Clinical Significance
Increased Acidity (Hyperchlorhydria):
-
Peptic ulcer disease (duodenal ulcer)
-
Zollinger-Ellison syndrome (gastrinoma)
-
Chronic gastritis (early stages)
-
Stress ulcers
Decreased Acidity (Hypochlorhydria):
-
Chronic atrophic gastritis
-
Helicobacter pylori infection
-
Gastric carcinoma
Absent Acidity (Achlorhydria):
-
Pernicious anemia (autoimmune destruction of parietal cells)
-
Advanced gastric carcinoma
-
Prolonged use of antacids or proton-pump inhibitors (PPIs)
Clinical Applications
| Condition | Free Acidity | Total Acidity | |
|---|---|---|---|
| Peptic Ulcer (Duodenal) | ↑↑ | ↑ | |
| Gastric Ulcer | Normal/↑ | Normal/↑ | |
| Pernicious Anemia | ↓↓ or 0 | ↓ | |
| Zollinger-Ellison Syndrome | ↑↑↑ | ↑↑ | |
| Gastric Carcinoma | ↓ | ↓ | |
| Use of PPIs/H2 blockers | ↓ | ↓ |