Introduction
-
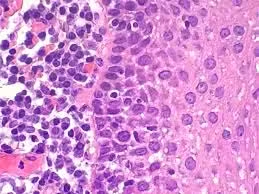
Hematoxylin and Eosin staining (H&E) is the most commonly used routine staining technique in histopathology.
-
It is the first-line stain applied for microscopic examination of tissue sections.
-
H&E staining provides excellent contrast between the nucleus and cytoplasm.
-
It allows clear visualization of cellular and tissue architecture.
-
Hematoxylin stains nuclei and other acidic components blue to purple.
-
Eosin stains cytoplasm and extracellular components pink to red.
-
The combined staining helps in identifying normal histology and pathological changes.
-
H&E staining is essential for the diagnosis of:
-
Inflammation
-
Necrosis
-
Degenerative changes
-
Tumors
-
-
It is a simple, rapid, and cost-effective staining method.
-
H&E staining forms the basis for further special stains and immunohistochemistry.
Principle of H&E Staining
-
The H&E staining technique is based on the differential affinity of tissue components for basic and acidic dyes.
-
Hematoxylin, after oxidation to hematein and in the presence of a mordant (usually aluminum or iron), acts as a basic dye.
-
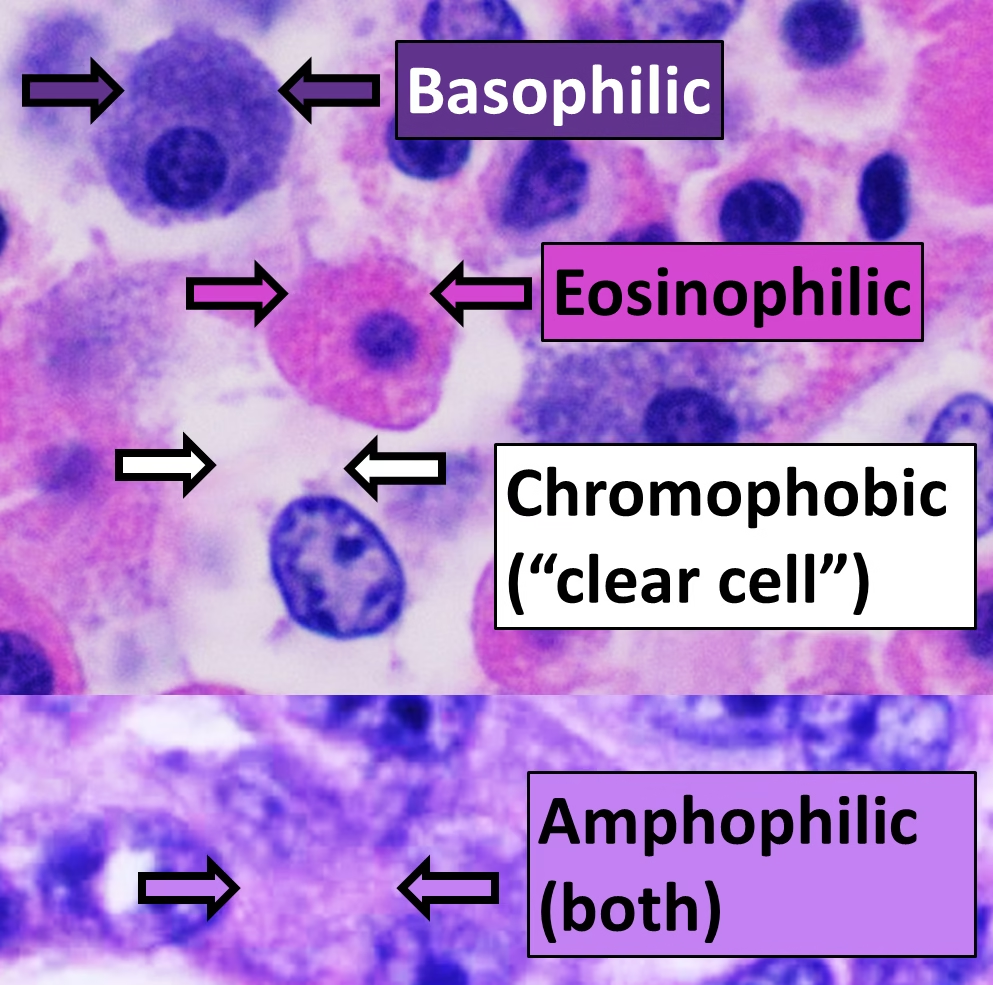
Hematoxylin binds to acidic (basophilic) structures of the cell, such as:
-
Nuclei
-
Chromatin
-
Ribosomes and rough endoplasmic reticulum
-
-
These structures are stained blue to purple.
-
Eosin is an acidic dye that binds to basic (acidophilic) components of tissues.
-
Eosin stains:
-
Cytoplasm
-
Collagen fibers
-
Muscle fibers
-
Red blood cells
-
-
These components appear pink to red.
-
The contrasting colors produced by hematoxylin and eosin allow clear visualization of tissue architecture and cellular details.
Components of H&E Stain
Components of H&E Stain
| Component | Source / Type | Function | Structures Stained / Role |
|---|---|---|---|
| Hematoxylin | Natural dye from Haematoxylum campechianum | Nuclear stain (after oxidation) | Stains nuclei, chromatin, nucleoli (blue–purple) |
| Hematein | Oxidized form of hematoxylin | Active staining compound | Binds to acidic tissue components |
| Mordant (Alum / Iron) | Aluminum or iron salts | Forms dye–mordant–tissue complex (lake) | Enhances nuclear staining and binding |
| Oxidizing agent | Sodium iodate / Mercuric oxide | Converts hematoxylin to hematein | Essential for staining action |
| Eosin (Eosin Y/B) | Synthetic acidic dye | Counterstain | Stains cytoplasm, collagen, muscle, RBCs (pink–red) |
| Differentiating agent | Acid alcohol | Removes excess hematoxylin | Sharpens nuclear details |
| Bluing agent | Tap water / Ammonia water / Scott’s water | Converts hematoxylin to blue color | Produces crisp nuclear staining |
Mechanism of Staining
Hematoxylin Staining
- Stains nuclei, ribosomes, and rough endoplasmic reticulum
- Colors range from blue to purple
Eosin Staining
- Stains cytoplasm, collagen, muscle fibers, and red blood cells
- Produces pink to red coloration
Staining Procedure
1. Deparaffinization
-
Paraffin wax is removed from tissue sections.
-
Slides are placed in xylene.
-
This step allows stains to penetrate the tissue.
2. Hydration
-
Slides are passed through descending grades of alcohol (100%, 95%, 70%).
-
Finally brought to water.
-
Necessary for proper hematoxylin staining.
3. Hematoxylin Staining
-
Slides are immersed in hematoxylin solution.
-
Nuclei and other basophilic structures take up the stain.
-
Nuclei appear blue to purple after subsequent steps.
4. Rinsing in Water
-
Excess hematoxylin is washed off.
-
Prepares tissue for differentiation.
5. Differentiation
-
Slides are treated with acid alcohol.
-
Removes excess hematoxylin from background tissue.
-
Sharpens nuclear details.
6. Bluing
-
Slides are placed in alkaline solution such as:
-
Tap water
-
Ammonia water
-
Scott’s tap water substitute
-
-
Converts hematoxylin to a stable blue color.
7. Eosin Staining
-
Slides are stained with eosin.
-
Cytoplasm and extracellular components take up pink coloration.
8. Dehydration
-
Slides are passed through ascending grades of alcohol.
-
Removes water from the tissue.
9. Clearing
-
Alcohol is replaced by xylene.
-
Makes tissue transparent and ready for mounting.
10. Mounting
-
Slides are mounted using a mounting medium.
-
A coverslip is placed to preserve the stained section.
Bluing Agents Used
- Tap water
- Ammonia water
- Scott’s tap water substitute
- Lithium carbonate
Types of Hematoxylin
- Harris hematoxylin
- Mayer’s hematoxylin
- Ehrlich’s hematoxylin
- Weigert’s hematoxylin
Microscopic Appearance of Tissues
| Tissue Component | Color | Staining Property |
|---|---|---|
| Nuclei | Blue–purple | Basophilic |
| Cytoplasm | Pink | Acidophilic |
| Collagen | Pale pink | Acidophilic |
| Muscle | Deep pink | Acidophilic |
| RBCs | Bright red | Acidophilic |
Advantages of H&E Staining
-
It is the most widely used routine stain in histopathology.
-
Provides excellent contrast between nucleus and cytoplasm.
-
Clearly demonstrates tissue architecture and cellular morphology.
-
Simple and easy to perform.
-
Cost-effective and economical for routine use.
-
Rapid staining procedure with short turnaround time.
-
Suitable for all types of tissues.
-
Serves as the first-line stain for diagnostic evaluation.
-
Helps in identifying:
-
Inflammation
-
Necrosis
-
Fibrosis
-
Tumors
-
-
Acts as a baseline stain before special stains and immunohistochemistry.
-
Produces reproducible and reliable results when properly performed.
Limitations of H&E Staining
-
It does not identify specific chemical components of tissues.
-
Cannot differentiate biochemically similar substances (e.g., collagen subtypes, mucins).
-
Limited specificity for microorganisms such as bacteria, fungi, and parasites.
-
Does not identify specific proteins, enzymes, or antigens.
-
Cannot accurately classify tumors without:
-
Special stains
-
Immunohistochemistry (IHC)
-
Molecular techniques
-
-
Subtle cellular components may be poorly visualized.
-
Interpretation depends heavily on:
-
Proper fixation
-
Quality of staining
-
Observer experience
-
-
Cannot assess functional or molecular alterations in tissues.
-
Some pathological conditions require additional confirmatory stains.
Quality Control in H&E Staining
-
Ensure proper fixation of tissue (adequate volume and duration of fixative).
-
Use well-processed, properly embedded tissue sections.
-
Cut sections of uniform thickness (usually 3–5 µm).
-
Use fresh, filtered, and correctly prepared hematoxylin and eosin solutions.
-
Monitor pH and strength of stains regularly.
-
Avoid overstaining or understaining by standardizing staining time.
-
Perform controlled differentiation to obtain crisp nuclear detail.
-
Ensure adequate bluing for stable blue nuclear staining.
-
Maintain proper alcohol and xylene quality for dehydration and clearing.
-
Prevent cross-contamination of reagents by regular replacement.
-
Run known control slides to assess staining consistency.
-
Check slides for:
-
Nuclear clarity
-
Cytoplasmic contrast
-
Background cleanliness
-
-
Maintain documentation and logs for stain preparation and QC checks.
-
Regularly train staff and follow standard operating procedures (SOPs).
-
Review stained slides before reporting to ensure diagnostic adequacy.
Common Errors and Artifacts
1. Overstaining with Hematoxylin
-
Nuclei appear very dark or obscured
-
Loss of nuclear detail
-
Caused by prolonged staining or inadequate differentiation
2. Understaining with Hematoxylin
-
Nuclei appear pale or faint
-
Poor nuclear visibility
-
Due to weak stain or insufficient staining time
3. Inadequate Bluing
-
Nuclei appear reddish-purple instead of blue
-
Caused by:
-
Insufficient alkaline treatment
-
Old or ineffective bluing agent
-
4. Overstaining with Eosin
-
Cytoplasm appears too dark or muddy
-
Masks cellular details
-
Occurs due to prolonged eosin staining
5. Understaining with Eosin
-
Cytoplasm appears very pale
-
Poor contrast between nucleus and cytoplasm
-
Caused by weak eosin or over-differentiation
6. Uneven Staining
-
Patchy or irregular staining across the section
-
Caused by:
-
Incomplete deparaffinization
-
Uneven reagent exposure
-
7. Nuclear Fading
-
Loss of nuclear staining over time
-
Due to:
-
Improper mounting
-
Use of acidic mounting medium
-
8. Tissue Shrinkage
-
Artificial spaces around cells or tissue distortion
-
Caused by:
-
Rapid dehydration
-
Prolonged exposure to alcohol or xylene
-
9. Section Lifting or Floating
-
Sections detach from slide during staining
-
Due to:
-
Poor slide adhesion
-
Inadequate drying
-
10. Precipitate Formation
-
Dark granules or deposits on slide
-
Caused by:
-
Unfiltered stains
-
Old hematoxylin solution
-
11. Air Bubbles
-
Clear round spaces under coverslip
-
Due to improper mounting technique
12. Knife Marks / Chatter
-
Parallel lines or vibration marks in section
-
Caused by microtome blade issues or hard tissue
MCQs
1. H&E staining is mainly used for:
A. Enzyme localization
B. Molecular diagnosis
C. Routine histopathological examination
D. Cytogenetics
✅ Answer: C
2. Hematoxylin is obtained from:
A. Coal tar
B. Logwood tree
C. Synthetic dye
D. Plant resin
✅ Answer: B
3. The active staining form of hematoxylin is:
A. Hematin
B. Hematein
C. Hemoglobin
D. Melanin
✅ Answer: B
4. Hematoxylin stains which tissue components?
A. Cytoplasm
B. Collagen
C. Acidic nuclear components
D. Lipids
✅ Answer: C
5. Eosin is classified as a:
A. Basic dye
B. Neutral dye
C. Acidic dye
D. Mordant
✅ Answer: C
6. Eosin primarily stains:
A. Nuclei
B. DNA
C. Basic tissue components
D. Acidic components
✅ Answer: C
7. Which structure appears blue in H&E stain?
A. Muscle fiber
B. Cytoplasm
C. Red blood cells
D. Nucleus
✅ Answer: D
8. Mordants are required in H&E staining to:
A. Oxidize eosin
B. Bind dye to tissue
C. Remove excess stain
D. Dehydrate tissue
✅ Answer: B
9. Commonly used mordant in hematoxylin is:
A. Sodium chloride
B. Potassium dichromate
C. Aluminum salt
D. Copper sulfate
✅ Answer: C
10. Bluing step converts hematoxylin color to:
A. Red
B. Pink
C. Blue
D. Yellow
✅ Answer: C
11. Which agent is used for bluing?
A. Acid alcohol
B. Xylene
C. Tap water
D. Formalin
✅ Answer: C
12. Differentiation in H&E staining removes excess:
A. Eosin
B. Hematoxylin
C. Paraffin
D. Mountant
✅ Answer: B
13. Acid alcohol is used for:
A. Fixation
B. Differentiation
C. Dehydration
D. Clearing
✅ Answer: B
14. The first step of H&E staining is:
A. Hydration
B. Hematoxylin staining
C. Deparaffinization
D. Mounting
✅ Answer: C
15. Deparaffinization is done using:
A. Alcohol
B. Water
C. Xylene
D. Acetone
✅ Answer: C
16. Cytoplasm appears _____ after H&E staining.
A. Blue
B. Purple
C. Pink
D. Green
✅ Answer: C
17. Red blood cells appear _____ in H&E stain.
A. Blue
B. Pale pink
C. Bright red
D. Purple
✅ Answer: C
18. Which hematoxylin is progressive in nature?
A. Harris
B. Ehrlich
C. Mayer’s
D. Weigert’s
✅ Answer: C
19. H&E staining is best described as:
A. Specific stain
B. Special stain
C. Routine stain
D. Enzyme stain
✅ Answer: C
20. H&E staining helps mainly in assessing:
A. Antigens
B. Tissue morphology
C. Enzyme activity
D. DNA sequence
✅ Answer: B
21. Pale nuclei in H&E indicate:
A. Overstaining
B. Understaining
C. Proper staining
D. Excess eosin
✅ Answer: B
22. Muddy cytoplasm is due to:
A. Understaining eosin
B. Overstaining eosin
C. Poor fixation
D. Excess bluing
✅ Answer: B
23. Nuclear fading occurs due to:
A. Over-bluing
B. Acidic mounting medium
C. Weak eosin
D. Thick sections
✅ Answer: B
24. Uneven staining is commonly due to:
A. Poor microscopy
B. Incomplete deparaffinization
C. Excess fixation
D. Over-bluing
✅ Answer: B
25. H&E staining does NOT identify:
A. Tumors
B. Inflammation
C. Tissue architecture
D. Specific antigens
✅ Answer: D
26. Section thickness for ideal H&E staining is:
A. 1–2 µm
B. 3–5 µm
C. 7–10 µm
D. 10–15 µm
✅ Answer: B
27. Which step follows eosin staining?
A. Bluing
B. Differentiation
C. Dehydration
D. Fixation
✅ Answer: C
28. Clearing agent used in H&E staining is:
A. Alcohol
B. Water
C. Xylene
D. Formalin
✅ Answer: C
29. H&E stain is inadequate alone for diagnosing:
A. Inflammation
B. Necrosis
C. Tumor typing
D. Tissue damage
✅ Answer: C
30. The main limitation of H&E staining is lack of:
A. Simplicity
B. Speed
C. Specificity
D. Contrast
✅ Answer: C
31. Which artifact is caused by unfiltered hematoxylin?
A. Air bubbles
B. Precipitate deposits
C. Section folding
D. Chatter
✅ Answer: B
32. H&E staining quality depends MOST on:
A. Microscope quality
B. Proper fixation
C. Coverslip thickness
D. Mountant color
✅ Answer: B
33. Which step stabilizes nuclear staining?
A. Differentiation
B. Bluing
C. Dehydration
D. Clearing
✅ Answer: B
34. H&E staining is essential before:
A. Grossing
B. Fixation
C. Immunohistochemistry
D. Embedding
✅ Answer: C
35. H&E staining remains important because it provides:
A. Molecular diagnosis
B. Genetic analysis
C. Baseline morphological assessment
D. Enzyme localization
✅ Answer: C