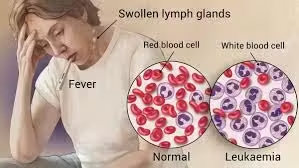
Leukaemia
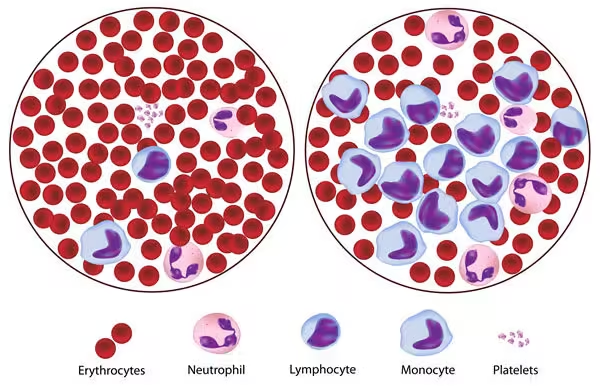
- Leukaemia is a type of cancer originating in the hematopoietic tissues, particularly the bone marrow, leading to the overproduction of abnormal white blood cells (leukocytes).
- These leukemic cells can be immature and dysfunctional, preventing the normal production of red blood cells, white blood cells, and platelets.
- As a result, patients with leukemia may experience various symptoms, including anemia, increased susceptibility to infections, and bleeding complications.
Pathophysiology
In leukemia, genetic mutations within hematopoietic stem cells result in the clonal proliferation of leukemic cells. These mutations may involve:
- Oncogenes: Genes that, when mutated or overexpressed, can promote cell growth and division.
- Tumor Suppressor Genes: Genes that normally inhibit cell division or promote apoptosis. Mutations can lead to uncontrolled cell proliferation.
- Epigenetic Changes: Modifications that affect gene expression without altering the DNA sequence, contributing to the leukemic phenotype.
The accumulation of these abnormal cells in the bone marrow leads to bone marrow failure, characterized by:
- Anemia: Due to a lack of red blood cell production.
- Thrombocytopenia: Resulting from reduced platelet production, increasing the risk of bleeding.
- Leukopenia: Potentially resulting from ineffective leukocyte maturation, causing an increased risk of infections despite a high white blood cell count.
Clinical Presentation
The clinical presentation of leukemia varies widely depending on the type and stage of the disease but often includes:
- Fatigue and Weakness: Due to anemia.
- Fever and Night Sweats: Resulting from underlying infections or cytokine release.
- Weight Loss: Often seen in advanced cases.
- Bone Pain: Due to bone marrow infiltration.
- Lymphadenopathy and Splenomegaly: Enlargement of lymph nodes and the spleen is common, especially in lymphoid leukemias.
- Bleeding and Bruising: Due to thrombocytopenia, leading to easy bruising, petechiae, or bleeding gums.
Classification of Leukemia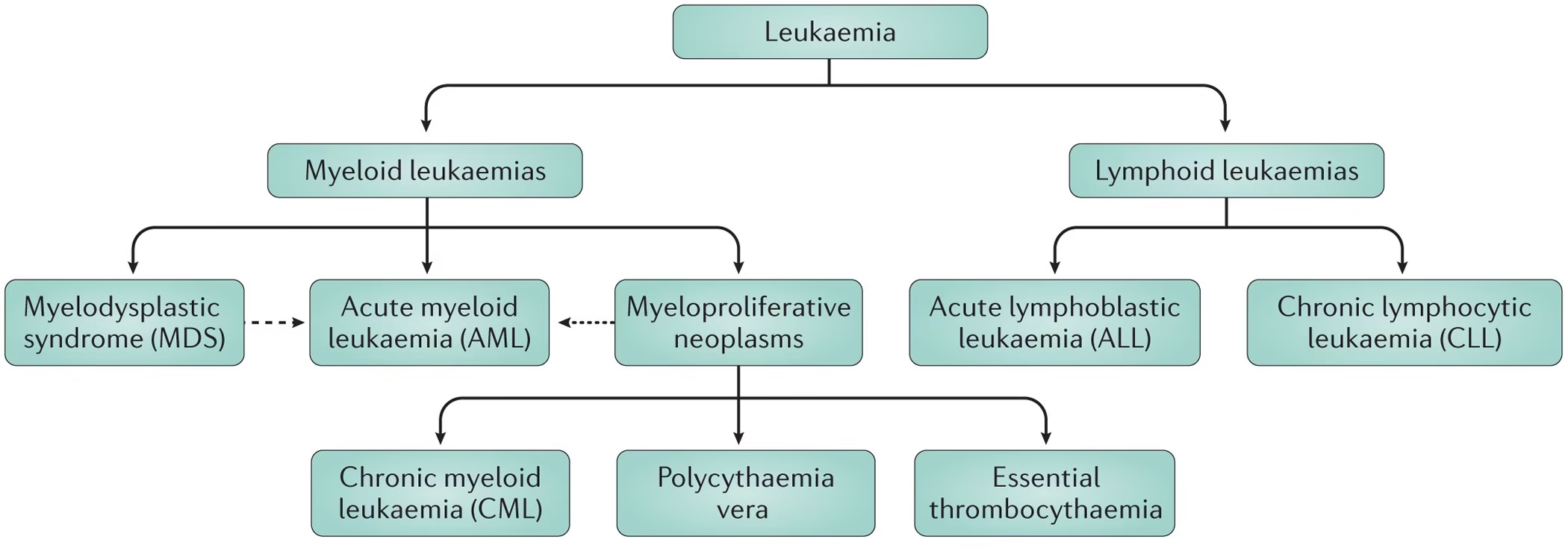
-
Classification Based on Progression Speed
- Acute Leukemia: Characterized by a rapid increase in immature cells (blasts). Symptoms typically develop quickly.
- Acute Lymphoblastic Leukemia (ALL): Common in children but can occur in adults. The proliferation of lymphoblasts characterizes it and is often associated with chromosomal abnormalities such as the Philadelphia chromosome or alterations involving chromosome 21.
- Acute Myeloid Leukemia (AML): More common in adults. It involves the myeloid lineage, with several subtypes classified based on the lineage and genetic abnormalities.
- Chronic Leukemia: Progresses more slowly, allowing patients to maintain normal function for an extended period.
- Chronic Lymphocytic Leukemia (CLL): The most common type of leukemia in adults involves accumulating small, mature lymphocytes. Often asymptomatic initially and diagnosed incidentally during routine blood tests.
- Chronic Myeloid Leukemia (CML) is characterized by the Philadelphia chromosome resulting from a translocation between chromosomes 9 and 22 (BCR-ABL fusion). It may present with splenomegaly and leukocytosis.
-
Classification Based on Cell Type
- Lymphoid Leukemias: Derived from lymphoid progenitor cells.
- ALL: Often presents with high blast counts and requires urgent treatment due to rapid disease progression.
- CLL: Typically has a good prognosis but can transform into more aggressive forms.
- Myeloid Leukemias: Derived from myeloid progenitor cells.
- AML: Can present with diverse clinical features depending on subtype, and treatment regimens vary based on specific genetic mutations.
- CML: Phases include chronic, accelerated, and blast phases, with treatment options evolving based on the phase.
-
Other Subtypes of Leukemia
- Hairy Cell Leukemia: A rare form of CLL characterized by “hairy” appearing B-cells. It often presents with splenomegaly and a specific pattern of cytopenia.
- Acute Promyelocytic Leukemia (APL): A subtype of AML associated with the promyelocytic leukemia (PML) gene on chromosome 15. It often requires treatment with all-trans retinoic acid (ATRA) and arsenic trioxide.
Laboratory Investigations for Leukemia
Complete Blood Count (CBC)
The CBC is a foundational test in the assessment of leukemia.
- White Blood Cell Count (WBC):
- Leukocytosis: Often seen in acute leukemias due to increased blasts.
- Leukopenia may be present in chronic leukemias or in patients with advanced disease.
- Hemoglobin (Hb): Usually low, indicating anemia.
- Platelet Count: Typically decreased in most types of leukemia.
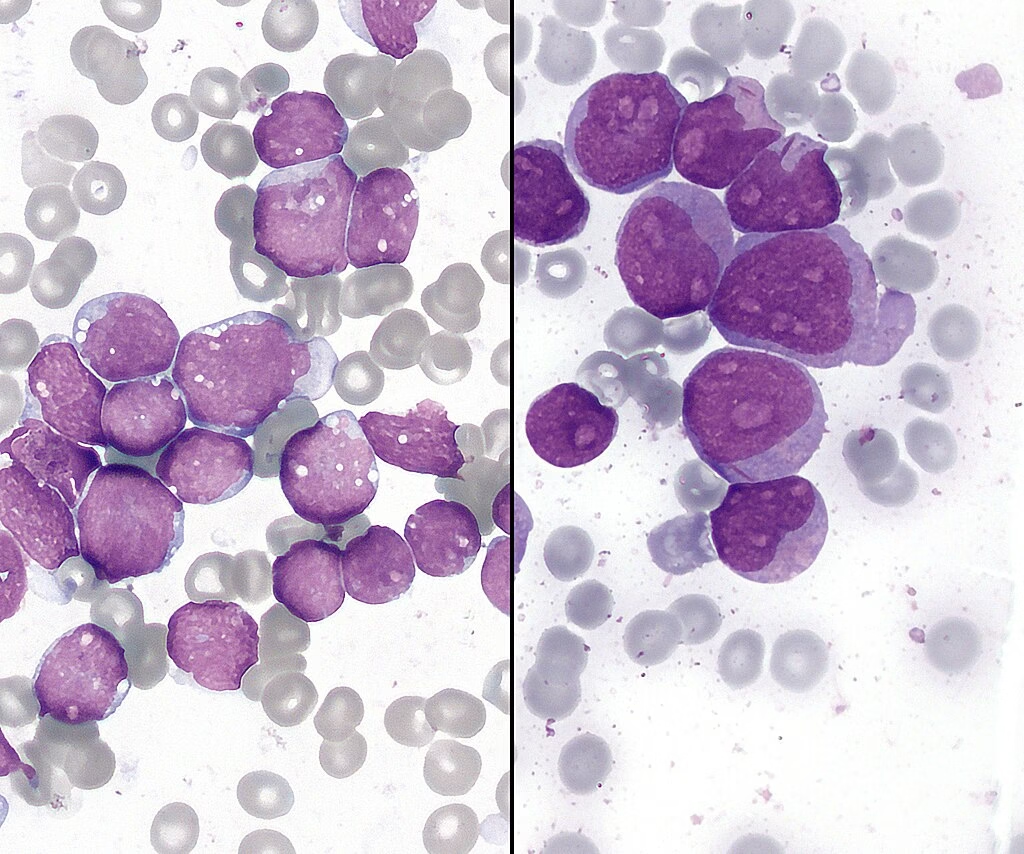
Peripheral Blood Smear
A peripheral blood smear provides crucial information about the morphology of blood cells.
- Blasts: The presence of a high percentage of blasts (≥20%) is indicative of acute leukemia.
- Cellular Morphology: Identification of specific features, such as:
- Auer Rods: Found in AML, indicating myeloid differentiation.
- Smudge Cells: Classic for CLL.
- Lymphoblasts: Indicators of ALL.
Bone Marrow Aspiration and Biopsy
This is critical for the diagnosis and classification of leukemia.
- Bone Marrow Aspirate: Evaluates cellularity and allows for cytological examination to assess the percentage of blasts.
- Bone Marrow Biopsy: A core sample that provides information on the architecture of the marrow and the extent of infiltration by leukemic cells. It can show hypercellularity, abnormal cell morphology, and fibrosis.
Cytogenetic Analysis
Cytogenetics is essential for the diagnosis, classification, and prognostication of leukemia.
- Karyotyping: Identifies chromosomal abnormalities, such as translocations or deletions. Common findings include:
- Philadelphia Chromosome: Found in CML (BCR-ABL fusion).
- t(15;17): Associated with APL.
- t(8;21) or inv(16): Indicate favorable prognostic subtypes of AML.
- Fluorescence In Situ Hybridization (FISH): Used for detecting specific genetic abnormalities and fusion genes with higher sensitivity than traditional karyotyping.
Immunophenotyping
Immunophenotyping through flow cytometry is a crucial step in leukemia diagnosis.
- Surface Markers: Allows to classify of leukemic cells based on surface antigens. Common markers include:
- CD19, CD20: B-cell markers (ALL, CLL).
- CD3, CD7: T-cell markers (T-ALL).
- Myeloid Markers (e.g., CD33, CD34): For AML classification.
- Identifying Blasts: Helps distinguish between different types of acute leukemias and informs treatment decisions.
Molecular Testing
Molecular testing is increasingly important for diagnosis and monitoring.
- Polymerase Chain Reaction (PCR): Detects specific mutations or fusion genes associated with leukemias. For example:
- BCR-ABL fusion in CML.
- FLT3-ITD mutations in AML.
- Next-Generation Sequencing (NGS): Provides a comprehensive profile of genetic mutations and alterations, facilitating targeted therapies.
Lactate Dehydrogenase (LDH)
- Elevated LDH: A marker of cell turnover and can indicate a high tumor burden. High levels of LDH are often associated with poor prognosis and aggressive disease.
Coagulation Studies
- Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT): Evaluated to check for coagulation abnormalities that can occur in leukemia, especially in acute forms that can lead to disseminated intravascular coagulation (DIC).
Other Laboratory Tests
- Serum Chemistry: Assesses liver and kidney function, which can be affected by leukemic processes or treatment complications.
- Cytokine Profile: Certain cytokines may be elevated in leukemia and can provide insight into disease activity and response to treatment.
Imaging Studies
- CT Scans May be utilized to assess lymphadenopathy, splenomegaly, or other organ involvement.
- Ultrasound: Useful for evaluating splenic enlargement.
Prognosis and Management
The prognosis and management of leukemia depend on various factors, including the specific type, genetic abnormalities, patient age, and overall health. Treatment options include:
- Chemotherapy: The mainstay of treatment for acute leukemias, typically administered in induction and consolidation phases.
- Targeted Therapy:
- Tyrosine Kinase Inhibitors (TKIs) for CML (e.g., imatinib).
- Monoclonal Antibodies for CLL (e.g., rituximab).
- Immunotherapy: Emerging options, particularly CAR T-cell therapy for ALL.
- Stem Cell Transplantation: Considered for eligible patients with high-risk leukemia or those who relapse after initial treatment.
- Supportive Care: Includes transfusions, antibiotics, and growth factors to manage complications.