Introduction
-
Urine is the final excretory product formed by the kidneys and reflects overall metabolic and renal function.
-
Normal urine composition consists of ~95% water and **~5% dissolved organic and inorganic solutes.
-
These solutes arise from normal metabolism, dietary intake, and renal regulatory mechanisms.
-
Major organic constituents include:
-
Urea (end product of protein and amino acid metabolism)
-
Creatinine (from muscle creatine breakdown)
-
Uric acid (from purine metabolism)
-
Hippuric acid, ammonia, and other minor metabolites
-
-
Important inorganic constituents include:
-
Electrolytes: Na⁺, K⁺, Cl⁻, HCO₃⁻
-
Phosphate, sulfate, Ca²⁺, Mg²⁺
-
Trace elements depending on diet and metabolism
-
-
Urine also normally contains small amounts of pigments (e.g., urochrome), giving it its characteristic yellow color.
-
Minor quantities of hormones, vitamins, and metabolic by-products may also be present, depending on physiological state.
-
The concentration of normal constituents varies with hydration status, diet, physical activity, and kidney function.
-
Studying normal urine composition provides the baseline for interpreting abnormalities in biochemical and clinical settings.
-
Any deviation from these normal constituents—such as presence of glucose, proteins, ketones, bilirubin, blood, or abnormal metabolites—indicates potential pathological conditions.
Urine collection
Urine is usually collected in a sterile, wide-mouthed container.
Different methods of urine collection are –
- First Morning Sample (Conc. Urine for Biochemical Analysis, Casts and Crystals)
- Random Sample (Chemical screening, Microscopic Examination)
- 24 Hour Urine Sample (Quantitative Estimation of Proteins, Sugars, Electrolytes, Hormones)
- Mid-Stream Urine
Sample preservation
- To determine urea, ammonia, nitrogen, and calcium, Hydrochloric acid is used.
- For the determination of sodium, potassium, chloride, bicarbonate, calcium, phosphorus, urea, ammonia, amino acids, creatinine, proteins, reducing substances, and ketone bodies, Thymol is used
- For the determination of Ascorbic acid, Acetic acid is used
- Toluene may also be used as a preservative.
Composition of normal urine
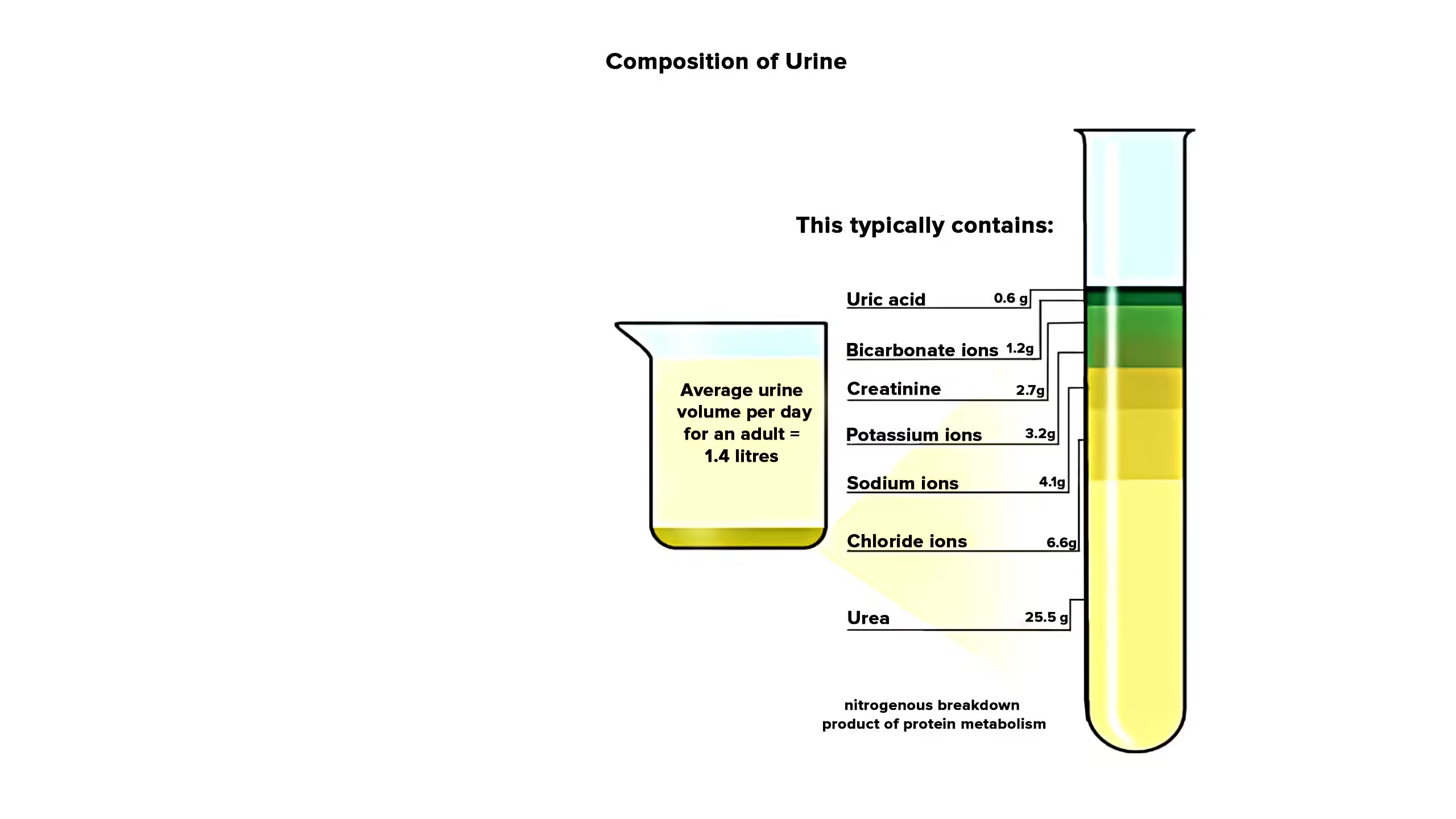
- Water – 90 – 95%
- Solid particles 5 – 10%
-
- Organic
- Inorganic
Properties of urine
Physical properties of urine
Volume
- The daily urine volume of normal subjects ranges from 700 to 2000 ml/day.
- Night urine output < 400ml.
Causes of Oliguria
- Prerenal causes – low blood pressure, shock, bleeding, fluid deprivation.
- Renal causes – Acute tubular necrosis, poisons causing renal damage, renal vascular disease.
- Post renal causes – Calculi, prostate enlargement.
Causes of Polyuria
- Excessive salt intake, diabetes mellitus
- Deficiency of antidiuretic hormone
- Excessive fluid intake– Intake of diuretics
Colour
- Normal urine is pale yellow (due to the presence of pigment urochrome)
- When the output of urine is low it appears deep yellow.
- Freshly voided urine is clear and transparent.
- Long-standing urine may become turbid. (Due to precipitation of phosphates)
- The urine may be dark yellow or brownish in fever because of concentration.
- In liver disease, the urine may be green, brown, or deep yellow due to bile pigments.
- Blood or haemoglobin develops a smoky to red colour.
- The urine is dark brown due to methemoglobin and homogentisic acid.
- Methylene blue gives the urine a green appearance.
- Strongly acid urine is pink due to the precipitation of uric acid salts.
Odour
- The normal odour of urine is aromatic.
- The odour is modified by the ingestion of certain foods or drugs.
- This is noticed after eating asparagus; the odour is due to methyl mercaptan.
- The ammoniacal smell of urine is due to the action of bacteria on urea.
- In ketosis, the odour of excreted acetone is detected.
pH
- The mixed sample of normal urine in 24 hours has a pH of 6.0. Individual samples vary from 4.6 to 8.0.
- The urine is acid in high protein intake because excess phosphate and sulfate are formed in the catabolism of protein.
- Acidity is also increased in acidosis and in fever.
- The urine becomes alkaline on standing due to the conversion of urea to ammonia and loss of CO2to air.
- The acidity of urine is increased after strenuous muscular exercise (elimination of lactic acid), by ingestion of ammonium salts of strong acids.
Specific gravity
- Specific gravity indicates the concentrating ability of the kidneys.
- Normal range: 1.012 – 1.030
- Measured using a urinometer, refractometer, and dipsticks.
- Increased specific gravity of more than 1.030 seen in
- Dehydration
- Diabetes mellitus
- Congestive heart failure
- Proteinuria
- Adrenal insufficiency
- Decreased specific gravity seen in
- Hypothermia
- Diuretic therapy
Appearance
Normal urine is clear (transparent).
Causes of cloudiness
- Pus cells (white blood cells) are clear in filtering
- Bacteria or fungi cleared by centrifugation
- Colloidal suspension of fat (as in chyluria)
Chemical properties of urine
Organic
- Urea
- Uric acid
- Creatinine
- Ethereal sulphate (organic)
- Urobilinogen
Inorganic
- Chloride
- Sulphate
- Calcium
- Inorganic phosphate
- Ammonia
Organic
Urea
- Daily excretion: 20-30 gm/day
- Urea is formed in the liver.
- The end product of protein metabolism.
Sodium Hypobromite Test –
2ml urine + a Few drops of sodium hypobromite solution.
Observation:
Brisk effervescence
Inference:
Hypobromite decomposes urea to give nitrogen gas.
Clinical significance – Increased
- Fever
- Diabetes Mellitus
Decreased
- Liver disease
- Nephritis
Urease Test
Method –
Label two test tubes as ‘TEST’ and ‘CONTROL’ Into
TEST:
5ml urea solution + 2ml well-mixed urease suspension. Into CONTROL: 5ml urea solution + 2ml urease suspension heated strongly.
Incubate both TEST and CONTROL at room temperature for 15- 20 minutes. In the end, add 2 drops of phenolphthalein to both tubes.
Observation
In TEST: Pink Colour
In CONTROL: No pink colour
Inference
Urease converts urea into ammonia and carbonic acid. Under the Ph of the reaction condition, ammonia and carbonic acid are converted to ammonium carbonate, pH goes above 9.5. Since the content is alkaline, phenolphthalein gives a pink colour.
Uric acid
- Daily excretion: 0.6-1gm/ day
- The end product of purine metabolism.
- Increased levels of uric acid in urine are Uricosuria
Phosphotungstic Acid Reduction Test
Method
2ml urine + a few drops of phosphotungstic acid + 20% sodium carbonate.
Observation
The blue colour is formed
Inference
Uric acid is a reducing agent in strong alkaline conditions. It reduces colourless phosphotungstic acid to tungsten blue.
Schiff’s Test
Method
Wet a filter paper with a few drops of ammoniacal silver nitrate solution. Add 1 or 2 drops of uric acid solution on the same paper.
Observation:
Black colour is formed
Inference:
Uric acid reduces ammoniacal silver nitrate to metallic silver, which is black.
Creatinine
- Daily excretion: 2gm/day in males, 1gm/day in females.
- Urinary creatinine is formed from muscle creatine.
Method –
Label 2 test tubes as ‘test’ and ‘control’.
Into Test: 2ml urine + 2ml saturated picric acid + few drops of 10% NaOH
Into Control: 2ml water + 2ml saturated picric acid + few drops of 10% NaOH
Observation –
Test – Orange Colour,
Control – Yellow Colour
Inference –
Creatinine reacts with an alkaline picrate solution to form orange creatinine picrate.
Urobilinogen
Increased urobilinogen concentration in urine is a sensitive index of liver dysfunction or haemolytic disorder.
- Urobilinogen is present in excessive amounts in prehepatic and hepatic jaundice.
- Urobilinogen is absent in urine in post-hepatic jaundice
Method –
5ml of freshly voided urine + 1ml Ehrlich reagent, mix. Let stand for 5 mins.
Observation –
A red colour is seen through the test tube’s mouth.
Inference –
Urobilinogen reacts with p-dimethyl amino benzaldehyde of the reagent to give a red colour.
Inorganic
Chloride
Normal chloride excreted in 24 hr urine sample is 8 – 15 g/ day (NaCl).
Method –
Take 2 ml of urine + 0.5 ml of conc. HNO3 + 1ml AgNo3.
Observation –
Curdy white precipitate
Inference –
Chloride is precipitated as AgCl with Ag No3 in the presence of HNO3.
Clinical significance – Increased
- Polydipsia
- Use of diuretics
- Addison’s disease
Deceased
- Excessive Sweating
- Fasting
- Diarrhoea
- Vomiting
- Diabetes Insipidus
- Infections
- Cushing’s Syndrome
Sulphate
Daily excretion: 1g/ day
Sulphur is derived from the catabolism of sulphur-containing amino acids.
Method –
2ml urine + 2ml Barium Chloride
Observation –
White precipitate
Inference –
Sulphate is precipitated as barium sulphate with barium chloride.
Clinical significance –
Increased
- Homocystinuria
- High protein diet cystinuria
Decreased
- Renal Impairment
Calcium
Daily excretion – 200mg/day
Method –
5ml urine + 5 drops of 1% acetic acid + 5ml potassium oxalate.
Observation:
A trace amount of white precipitate
Inference:
Calcium is precipitated as calcium oxalate in an acid medium.
Clinical significance –
Increased
- Hyper Parathyroidism
- Renal Stones
- Hypervitaminosis D
Decreased
- Tetany
Inorganic phosphate
Daily excretion: 1gm/ day
Method –
5ml urine + a few drops of conc. HNO3+ a pinch of ammonium molybdate. Warm
Observation –
Canary yellow precipitate.
Inference:
Inorganic phosphate is precipitated as canary yellow ammonium phosphomolybdate.
Clinical significance –
Increased
- Rickets / Osteomalacia
- Hyperparathyroidism
- Acidosis
Decreased
- Diarrhoea
- Pregnancy
- Nephritis
Ammonia
Daily excretion: 0.5-0.8gm/ day
Urinary ammonia is derived from glutamine and other amino acids by the kidney.
Method –
5ml urine + 2% sodium carbonate (till the red litmus turns blue). Boil. During boiling, hold a piece of moistened red litmus paper at the mouth of the test tube.
Observation:
Red litmus turns blue
Inference:
Ammonia liberated turns red litmus blue.
Clinical significance –
Increased
- Diabetic Ketoacidosis
- Urinary Tract Infection
Decreased
- Alkalosis
- Nephritis