
- Nosocomial infections or hospital-acquired infections (HAIs), are infections patients acquire during their stay in healthcare facilities, typically within 48 hours of admission or 30 days of receiving healthcare interventions.
- These infections are significant public health concerns, as they increase morbidity, mortality, length of hospital stays, and healthcare costs.
- Key pathogens involved in nosocomial infections include Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
- This essay discusses nosocomial infections, the importance of sterility testing for intravenous (IV) fluids, and methods for processing various clinical samples for hospital infection control.
Nosocomial Infections:
- Nosocomial infections (healthcare-associated infections or HAIs) occur in patients during their stay in a healthcare facility and are not present or incubating at admission.
- They result in significant challenges for healthcare systems due to their impact on patient outcomes, particularly when caused by drug-resistant pathogens.
Causes and Pathogenesis
- Direct Transmission: Pathogens can be transmitted directly with healthcare workers, equipment, or other infected patients.
- Indirect Transmission: Environmental surfaces, contaminated medical devices, and healthcare workers’ hands can indirectly transfer pathogens to patients.
- Endogenous Infection: Patients may develop infections from their microbiota, which becomes pathogenic due to invasive procedures or compromised immunity.
- Opportunistic Infections: Weakened immunity from illness, surgery, or treatment (e.g., immunosuppressive drugs) makes patients more susceptible to infections from opportunistic pathogens.
Specific Nosocomial Infections
- Catheter-Associated Urinary Tract Infections (CAUTIs):
- Mechanism: Bacteria colonize or travel along the catheter to enter the bladder.
- Pathogens: Common pathogens include Escherichia coli, Enterococcus, and Pseudomonas aeruginosa.
- Prevention: CAUTIs can often be prevented by minimizing catheter use, maintaining catheter hygiene, and removing the catheter as soon as possible.
- Surgical Site Infections (SSIs):
- Mechanism: Contaminated surgical tools, poor skin preparation, or bacterial translocation from the skin can lead to infections.
- Pathogens: Staphylococcus aureus (including MRSA), Enterobacteriaceae, and anaerobic bacteria are frequently involved.
- Prevention: Strict sterilization of instruments, proper wound care, and prophylactic antibiotics help reduce SSIs.
- Ventilator-Associated Pneumonia (VAP):
- Mechanism: Intubation allows pathogens to bypass the upper airway defenses, reaching the lower respiratory tract.
- Pathogens: Pathogens include Pseudomonas aeruginosa, Acinetobacter baumannii, and Staphylococcus aureus.
- Prevention: Strategies include minimizing ventilation duration, elevating the head of the bed, and maintaining oral hygiene.
- Central Line-Associated Bloodstream Infections (CLABSIs):
- Mechanism: Pathogens enter the bloodstream through central venous catheters, particularly when the line is handled frequently.
- Pathogens: Staphylococcus aureus, Staphylococcus epidermidis, Candida spp., and Enterobacteriaceae are common culprits.
- Prevention: Proper catheter insertion techniques, sterilization, and care are essential to prevent CLABSIs.
- Clostridium difficile Infections (CDIs):
- Mechanism: Antibiotics disrupt gut flora, allowing C. difficile to overgrow, producing toxins that damage the intestinal lining.
- Pathogens: Clostridium difficile produces toxins A and B, causing colitis.
- Prevention: Limiting antibiotic use, proper hand hygiene, and isolation of infected patients help control the spread of C. difficile.
Sterility Testing of IV Fluids
Sterility testing of IV fluids is crucial as they are directly introduced into the bloodstream. Any contamination could lead to sepsis, endocarditis, or severe adverse events, especially in immunocompromised patients.
Sterility Testing Standards
- USP <71>: The United States Pharmacopeia (USP) outlines sterility testing standards, specifying that sterility testing should confirm the absence of any microbial contaminants in parenteral preparations.
- Ph. Eur.: The European Pharmacopoeia provides similar guidelines for sterility testing, emphasizing testing for bacterial, fungal, and mold contamination.
Techniques
- Membrane Filtration:
- Process: This involves filtering the IV fluid through a sterile membrane with pores small enough to trap microorganisms.
- Advantages: It’s efficient for large-volume samples and works well with clear fluids.
- Limitations: Fluids that are viscous or contain particulates can clog the filter and complicate testing.
- Direct Inoculation:
- Process: A sample is directly injected into nutrient-rich media and incubated for a specific period.
- Advantages: This method is simpler than filtration and ideal for viscous or particulate-containing solutions.
- Limitations: Requires careful handling to prevent contamination.
- Automated and Rapid Methods:
- ATP Bioluminescence: ATP testing measures microbial ATP, providing a rapid estimate of viable cells.
- Fluorescence-Based Techniques: These detect cell wall components like peptidoglycan or genetic markers.
- Advantages: These methods provide faster results than traditional techniques but are typically more costly and require specialized equipment.
Key Points in Sterility Testing for IV Fluids
- Environmental Monitoring: Sterility testing must consider environmental cleanliness in production and testing areas.
- Validation of Procedures: The sterility testing methods must be validated to ensure reliability and compliance.
- Interpretation of Results: Detecting growth in a single sample requires investigation and potential recall to prevent patient harm.
Processing Samples for Hospital Infection Control
Processing clinical samples for hospital infections involves identifying pathogens, tracking antibiotic resistance, and implementing measures to contain and eradicate infectious agents.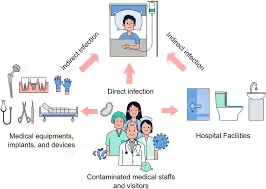
Sample Processing Steps
- Blood Cultures:
- Automated Blood Culture Systems: Incubate and detect microbial growth using colorimetry or fluorometry to indicate metabolic activity.
- Post-Detection Identification: Sub-culturing on media (e.g., blood agar) or using rapid identification systems like MALDI-TOF allows quick pathogen identification.
- Antibiotic Sensitivity Testing: Automated systems or disk diffusion methods determine resistance profiles.
- Urine Cultures:
- Plating and Incubation: Samples are plated on media like CLED or MacConkey agar to promote the growth of urinary pathogens.
- Automated Microscopy or Flow Cytometry: Detects bacterial counts, leukocytes, and other indicators of infection, allowing for more rapid diagnosis.
- Identification of Pathogens: Automated analyzers can identify common uropathogens quickly, expediting the process.
- Wound and Surgical Site Cultures:
- Sample Collection: Swabs or biopsies are plated on aerobic and anaerobic media for a broader pathogen spectrum.
- Pathogen Identification: Identification relies on traditional culture techniques or rapid molecular assays for critical pathogens, such as MRSA.
- Respiratory Samples:
- Sample Collection and Quality Control: Sputum samples undergo Gram staining to assess sample quality (e.g., low epithelial cells and high leukocytes indicate a good sample).
- Selective Media: Blood agar, chocolate agar, and selective agar (for gram-negative rods) help grow respiratory pathogens.
- Molecular Tests: PCR assays for organisms like Mycobacterium tuberculosis or Legionella spp. Aid in diagnosing specific infections.
- Stool Cultures:
- Selective and Differential Media: Media like XLD or MacConkey agar help isolate enteric pathogens.
- Toxin Detection: Enzyme immunoassays (EIAs) or PCR assays detect C. difficile toxins in suspected infections.
Infection Control and Surveillance Programs
- Effective infection control and surveillance programs are critical in reducing HAIs.
- These programs focus on monitoring infection rates, implementing preventive strategies, and educating healthcare staff.
Components
- Surveillance and Reporting: Hospitals establish systems to track infections, collect data, and report outcomes to health authorities. Automated infection surveillance systems help by providing real-time data analysis and alerts.
- Hand Hygiene Programs: Hand hygiene compliance is essential, with regular training, audits, and feedback for healthcare staff.
- Isolation Protocols: Strict isolation and contact precautions are used for patients infected or colonized with highly contagious or resistant pathogens.
- Environmental Cleaning: Regular disinfection of patient areas, high-touch surfaces, and equipment is essential to prevent cross-contamination.
- Antibiotic Stewardship: By monitoring antibiotic usage and restricting certain drugs, hospitals can prevent the development of resistant strains and limit unnecessary antibiotic exposure.
Challenges and Future Directions in Infection Control
- Emerging Multi-Drug Resistance: Increased antibiotic-resistant strains (e.g., carbapenem-resistant Enterobacteriaceae, MRSA) complicate treatment, necessitating innovative approaches like bacteriophage therapy or new antibiotic classes.
- Patient Education: Educating patients on hygiene and self-care during hospitalization can reduce infection risks.
- Advanced Surveillance Technologies: Incorporating AI and machine learning in surveillance systems can provide predictive analytics for infection outbreaks, allowing for proactive intervention.