
Introduction
-
Osmosis is the movement of water or solvent molecules from a region of low solute concentration to a region of high solute concentration through a semipermeable membrane.
-
It is a passive process that occurs without the use of energy (ATP) and helps maintain balance between two solutions.
-
The semipermeable membrane allows solvent molecules to pass while restricting the movement of solutes.
-
Osmosis plays a vital role in maintaining cell volume and overall fluid balance within the body.
-
It is essential for various biological processes, including nutrient absorption, kidney function, and water regulation within cells.
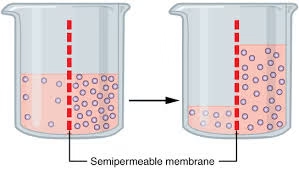
Basic Concept of Osmosis
- Osmosis is a type of passive transport, meaning it requires no external energy.
- It is driven purely by differences in osmotic pressure, which is the pressure needed to stop solvent movement through the membrane.
Mathematically, osmotic pressure (π) is given by van’t Hoff’s law:
π=iCRT
where:
-
-
i = ionization constant
-
C = molar concentration of solute
-
R = universal gas constant
-
T = absolute temperature (Kelvin)
-
This equation reflects how osmotic pressure is proportional to solute concentration and temperature.
Semipermeable Membrane and Its Role
A semipermeable membrane allows the passage of solvent molecules but restricts solutes. In biological systems, the plasma membrane plays this role, consisting of a phospholipid bilayer interspersed with proteins.
-
Aquaporins (specialized protein channels) facilitate rapid water transport.
-
Lipid solubility and membrane charge also influence the rate of osmosis.
Thus, membrane selectivity ensures that essential ions and macromolecules are retained while maintaining water balance.
Types of Osmotic Environments
Osmosis can cause cells to gain, lose, or maintain water depending on the external solution:
| Environment | Solute Concentration (Outside vs Inside) | Effect on Cell |
|---|---|---|
| Isotonic | Equal | No net water movement; cell volume stable |
| Hypotonic | Lower outside solute concentration | Water enters the cell; the cell swells (may lyse) |
| Hypertonic | Higher outside solute concentration | Water leaves the cell; the cell shrinks (crenation/plasmolysis) |
In human biochemistry, maintaining isotonic conditions (e.g., 0.9% NaCl for red blood cells) is crucial to prevent osmotic damage.

Factors Affecting Osmosis
Osmosis Role in Biochemistry
Osmosis is critical in many biochemical processes, especially in maintaining cellular function and homeostasis. Some key uses in biochemistry include:
- Cellular Homeostasis:
-
- Regulation of Cell Volume: Cells use osmosis to control their internal water content and volume. In hypotonic environments, water enters cells; in hypertonic environments, water exits, helping cells adapt to changes in their surroundings.
- Osmoregulation: Organisms regulate osmotic pressure to maintain fluid balance and prevent excessive water loss or gain.
- Transport of Nutrients and Waste:
-
- Nutrient Absorption: In tissues, osmosis assists in nutrient uptake and distribution by moving water and dissolved substances across cell membranes.
- Waste Removal: Osmosis helps eliminate metabolic wastes, especially in organisms that rely on diffusion for waste excretion.
- Kidney Function:
-
- Osmosis is crucial in the kidneys during urine formation, particularly in the nephrons (functional kidney units).
- Here, water moves by osmosis through membranes in response to varying solute concentrations, helping regulate water balance and filter out waste.
- Maintaining Blood Pressure:
-
- The osmotic movement of water between blood plasma and surrounding tissues influences blood pressure.
- This is regulated by albumin and other proteins in the bloodstream that exerts osmotic pressure, retaining water in the circulatory system.
- Energy Production in Cells:
-
- In cellular respiration, mitochondria rely on osmosis to generate ATP.
- The movement of protons (H⁺) across the mitochondrial membrane is an example of how osmosis can drive biochemical processes, specifically during the chemiosmotic mechanism of ATP synthesis.
MCQs
-
Osmosis is the movement of _______.
a) Solute from low to high concentration
b) Solvent from low to high solute concentration
c) Solvent from high to low solute concentration
d) Solute through a non-permeable membrane -
The process of osmosis requires a _______.
a) Permeable membrane
b) Semipermeable membrane
c) Non-permeable wall
d) Metallic barrier -
Osmosis is an example of _______ transport.
a) Active
b) Passive
c) Facilitated
d) Vesicular -
Osmotic pressure is the pressure required to _______.
a) Stop osmosis
b) Increase osmosis
c) Start diffusion
d) Dilute the solvent -
Which law explains osmotic pressure in dilute solutions?
a) Boyle’s Law
b) Charles’ Law
c) van’t Hoff’s Law
d) Graham’s Law -
Osmotic pressure is directly proportional to _______.
a) Solute concentration and temperature
b) Solvent volume only
c) Membrane surface area
d) Solvent density -
The equation for osmotic pressure is:
a) π = iCRT
b) π = mgh
c) π = RT/C
d) π = PV/n -
The movement of solvent in osmosis continues until _______.
a) Equilibrium is reached
b) Solute dissolves completely
c) Energy is consumed
d) Temperature decreases -
In biological systems, the main solvent involved in osmosis is _______.
a) Alcohol
b) Water
c) Glucose
d) Urea -
A solution with lower solute concentration compared to the cell interior is called _______.
a) Isotonic
b) Hypotonic
c) Hypertonic
d) Neutral -
Cells placed in a hypertonic solution will _______.
a) Swell
b) Shrink
c) Remain the same
d) Burst -
In a hypotonic solution, a red blood cell will _______.
a) Shrink
b) Burst (hemolyze)
c) Remain unchanged
d) Lose color -
The plasma membrane acts as a _______ in osmosis.
a) Permeable barrier
b) Semipermeable membrane
c) Non-conductive wall
d) Impermeable barrier -
Osmosis differs from diffusion because it involves _______.
a) Solvent movement through semipermeable membrane
b) Solute movement in air
c) Active transport of molecules
d) Energy utilization -
Which of the following affects the rate of osmosis?
a) Temperature
b) Concentration gradient
c) Membrane permeability
d) All of the above -
The osmotic pressure of pure water is _______.
a) Zero
b) Maximum
c) Equal to 1 atm
d) Negative -
Osmosis helps in maintaining _______ in living cells.
a) Temperature
b) Pressure balance and cell volume
c) Enzyme activity
d) DNA stability -
The process of osmosis in plants helps in _______.
a) Photosynthesis
b) Water absorption and turgor pressure maintenance
c) Seed germination
d) Respiration -
When a cell is in an isotonic solution, water movement is _______.
a) Only into the cell
b) Only out of the cell
c) Equal in both directions
d) Completely stopped -
Reverse osmosis occurs when _______.
a) Pressure greater than osmotic pressure is applied
b) Solvent moves naturally
c) Solute is non-diffusible
d) Temperature decreases -
Reverse osmosis is used for _______.
a) Water purification
b) Gas separation
c) Protein synthesis
d) DNA replication -
Osmosis stops when _______.
a) Osmotic pressure equals applied pressure
b) Solute dissolves fully
c) Membrane becomes impermeable
d) Cell bursts -
Osmosis can be demonstrated by using _______.
a) Potato cup experiment
b) Paper chromatography
c) Centrifugation
d) Gel electrophoresis -
Which of the following is a colligative property?
a) Osmotic pressure
b) Surface tension
c) Viscosity
d) Density -
Osmotic pressure depends on _______.
a) Number of solute particles
b) Type of solute
c) Shape of solute
d) Color of solution -
The unit of osmotic pressure is _______.
a) Joule
b) Atmosphere (atm) or Pascal (Pa)
c) Liter
d) Newton -
Osmosis is essential in kidneys for _______.
a) Urea synthesis
b) Water reabsorption
c) Protein digestion
d) Bile secretion -
The rate of osmosis increases when _______.
a) Solute concentration difference increases
b) Membrane thickness increases
c) Temperature decreases
d) Pressure is reduced -
The process opposite to osmosis is _______.
a) Active transport
b) Reverse osmosis
c) Diffusion
d) Filtration -
The osmotic pressure of a 1 M NaCl solution at 25°C (assuming ideal behavior) will be approximately _______.
a) 22.4 atm
b) 49.2 atm
c) 24.5 atm
d) 2 atm -
Osmotic balance between blood and tissue fluid prevents _______.
a) Hemolysis or crenation
b) Clot formation
c) Enzyme activation
d) DNA damage -
When water enters a plant cell, it becomes _______.
a) Plasmolyzed
b) Turgid
c) Flaccid
d) Dead -
The condition where a cell loses water in a hypertonic environment is called _______.
a) Plasmolysis
b) Turgidity
c) Hemolysis
d) Crystallization -
Osmosis plays a vital role in maintaining _______.
a) Homeostasis
b) Bone density
c) Nerve conduction
d) Vision -
Aquaporins are _______.
a) Protein channels for water movement
b) Enzymes that digest solutes
c) Lipid molecules
d) Ion pumps
✅ Answer Key
-
b
-
b
-
b
-
a
-
c
-
a
-
a
-
a
-
b
-
b
-
b
-
b
-
b
-
a
-
d
-
a
-
b
-
b
-
c
-
a
-
a
-
a
-
a
-
a
-
a
-
b
-
b
-
a
-
b
-
b
-
a
-
b
-
a
-
a
-
a