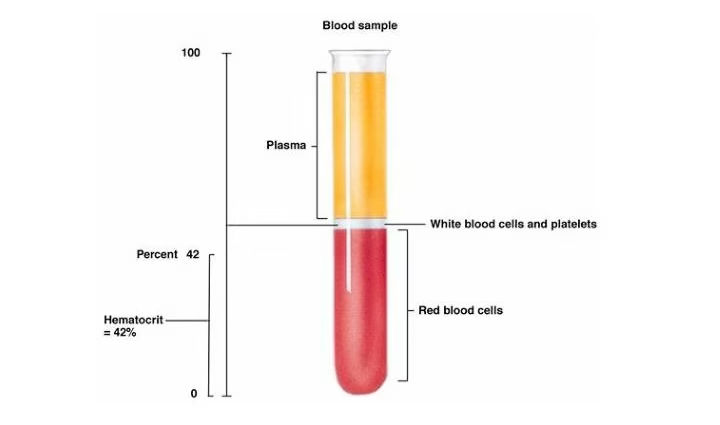
AIM: Determination of PCV (Packed Cell Volume), also known as hematocrit (HCT)
Microhematocrit Method
Principle
- The principle of the microhematocrit method is based on the separation of blood components by centrifugation.
- When blood is centrifuged, the red blood cells, being denser, settle at the bottom of the capillary tube, forming the “packed” layer, while plasma, the less dense component, stays at the top.
- The ratio of the volume of packed red blood cells to the total blood volume is expressed as a percentage, representing the hematocrit or PCV.
Requirements
- Anticoagulated blood: EDTA blood
- Capillary (Microhematocrit) tubes
- Sealing clay
- Microhematocrit centrifuge
- Microhematocrit reader
Procedure
- Blood Collection:

- Use an anticoagulated blood sample (EDTA or heparinized blood) or directly collect capillary blood (e.g., from a fingerstick) into the microhematocrit tube.
- Filling the Capillary Tube:
- Fill the microhematocrit tube about two-thirds to three-fourths full by capillary action or by gently dipping the tube into the blood sample.
- Sealing:
- Seal one end of the capillary tube with sealing clay to prevent blood from escaping during centrifugation.
- Centrifugation:
- Place the sealed tube in the microhematocrit centrifuge with the sealed end facing outward.
- Centrifuge the tubes at 10,000–15,000 RPM for about 5 minutes to separate the blood components into distinct layers.
- Bottom layer: Packed red blood cells (RBCs).
- Middle layer: Buffy coat (white blood cells and platelets).
- Top layer: Plasma (clear or yellowish fluid).
- Reading the PCV:
- After centrifugation, use a microhematocrit reader to measure the height of the red blood cell column and the total height of the blood column.
- Express the PCV as a percentage (e.g., 45% or 0.45).
Interpretation of Results
- Normal PCV Values:
- Men: 40-54% (0.40-0.54)
- Women: 36-48% (0.36-0.48)
- Children: 35-45% (0.35-0.45)
- Newborns: 44-64% (0.44-0.64)
- High PCV (Polycythemia): Indicates increased red blood cell production or reduced plasma volume (as seen in dehydration or chronic hypoxia).
- Low PCV (Anaemia): Indicates a reduction in red blood cell count or total blood cell mass due to various causes like nutritional deficiencies, blood loss, or bone marrow disorders.
Advantages of the Microhematocrit Method
- Requires only a small blood volume: Useful in pediatric cases or when venous blood is difficult to obtain.
- Quick and cost-effective: Ideal for routine or point-of-care testing.
- Simple setup: This can be performed in laboratories with minimal equipment.
Disadvantages
- Manual reading may introduce errors: Accurate reading of PCV depends on the operator’s skill.
- May not provide detailed information: The method does not specify the causes of abnormal PCV levels (e.g., the cause of anaemia or polycythemia).
Clinical Significance
- The microhematocrit method is important in diagnosing conditions like:
- Anaemia: Low PCV values suggest reduced red blood cell count or mass.
- Polycythemia: High PCV values indicate an increased red cell mass, often due to chronic lung disease, heart conditions, or dehydration.
- Dehydration: Causes falsely elevated PCV due to reduced plasma volume.
- Overhydration or pregnancy: This can lead to low PCV due to increased plasma volume.
Macro Method (Wintrobe)
Principle
- The principle of the macro method, like the micro method, is based on the separation of blood components by centrifugation.
- After spinning an anticoagulated blood sample in a Wintrobe tube, the blood separates into three layers: red blood cells (RBCs) at the bottom, a thin “buffy coat” of white blood cells (WBCs) and platelets in the middle, and plasma at the top.
- The percentage of the total blood volume occupied by red blood cells is the PCV.
Requirements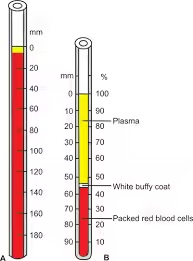
- Wintrobe tube
- Anticoagulated (EDTA) blood sample
- Centrifuge
- Wintrobe tube reader or ruler
Procedure
- Filling the Wintrobe Tube:
- Fill the Wintrobe tube up to the zero mark (approximately 10 cm) with the anticoagulated blood sample.
- The tube is typically marked from 0 to 100 mm, allowing for direct measurement of the packed red blood cell column.
- Centrifugation:
- Place the Wintrobe tube in a centrifuge and spin it at 2,300–3,000 RPM for about 30 minutes.
- This allows for completely separating red blood cells from the plasma.
- Reading the PCV:
- After centrifugation, three layers will be visible in the tube:
- Bottom layer: Packed red blood cells (RBCs).
- Middle layer: Buffy coat (thin layer of WBCs and platelets).
- Top layer: Plasma.
- Measure the height of the red blood cell column relative to the total blood column using the calibrations on the Wintrobe tube.
- The result is a percentage or decimal value representing the PCV.
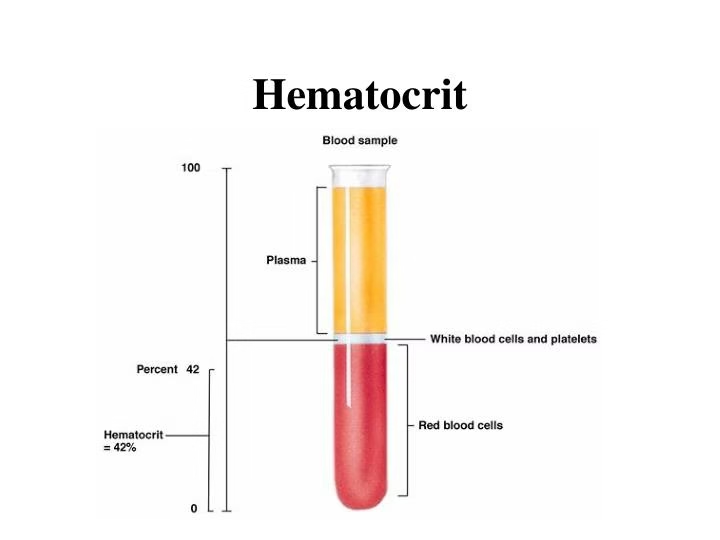
- After centrifugation, three layers will be visible in the tube:
Normal PCV Values for the Macro Method
The normal values for PCV are similar regardless of the method used:
- Men: 40-54% (0.40-0.54)
- Women: 36-48% (0.36-0.48)
- Children: 35-45% (0.35-0.45)
- Newborns: 44-64% (0.44-0.64)
Clinical Significance
- High PCV:
- Polycythemia Vera: A condition in which the bone marrow produces too many red blood cells, increasing blood viscosity.
- Dehydration: Loss of plasma volume increases PCV, leading to higher readings.
- Chronic Hypoxia: Chronic obstructive pulmonary disease (COPD) or living at high altitudes can cause increased red cell production as a compensatory mechanism for low oxygen levels.
- Low PCV:
- Anaemia: Caused by reduced red blood cell production, increased red blood cell destruction, or blood loss.
- Nutritional Deficiencies: Low iron, vitamin B12, or folate levels can decrease red cell production.
- Overhydration: Dilution of the blood plasma reduces the percentage of red blood cells, resulting in a low PCV.
Advantages
- Accuracy: The macro method is more precise than the micro method because it uses a larger blood volume, reducing random errors.
- Simultaneous ESR Measurement: The same Wintrobe tube can measure erythrocyte sedimentation rate (ESR), making diagnosing inflammatory conditions and PCV convenient.
- Larger Sample Volume: This method allows for additional analyses from the same sample.
Disadvantages
- Longer Processing Time: The centrifugation process takes longer (about 30 minutes) than the micro method (5 minutes).
- Larger Blood Volume: Requires a greater blood volume, which may be a limitation for pediatric or severely anaemic patients.
- Manual Labor: The method is more labour-intensive and time-consuming than automated hematocrit methods.