
Introduction
Surface tension is the force that causes the surface of a liquid to behave like a stretched elastic membrane.
It arises due to cohesive forces between liquid molecules, particularly hydrogen bonding in water.
Definition
Surface tension is defined as:
“The amount of work required to increase the surface area of a liquid by one unit area.”
Or
“Force acting along a line of 1 cm on the surface of a liquid.”
Measured in: dynes/cm or mN/m
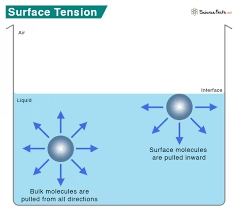
Why Surface Tension Is High in Water?
Water has an unusually high surface tension (~72 dynes/cm) due to:
1. Strong Hydrogen Bonding
-
Each water molecule forms up to 4 hydrogen bonds.
-
Creates a dense network of cohesive forces.
2. Dipole-Dipole Interactions
-
The polar nature of H₂O creates electrostatic attraction.
3. Molecular Cohesion Greater Than Adhesion
-
Strong attraction between water molecules compared to the surrounding air.

Surface Tension and Temperature
Surface tension decreases with increasing temperature because:
-
Heat disrupts hydrogen bonding
-
Molecular movement increases
-
Cohesive forces weaken
This principle is essential in physiology – e.g., surfactant decreases surface tension more efficiently at low lung volumes.
Importance of Surface Tension
Surface tension plays a vital role in multiple biochemical processes:
A. Role in Biological Membranes
-
Cell membranes form due to hydrophobic interactions, reducing surface free energy.
-
Phospholipids align into bilayers because this configuration minimizes surface tension between water and hydrophobic tails.
B. Protein Structure & Enzyme Function
-
Hydrophobic amino acids move toward the protein core, minimizing surface energy.
-
Hydrophilic amino acids position at the surface to interact with water.
This drives protein folding, stability, and molecular recognition.
C. Pulmonary Surfactant System – A Classic Biochemical Application
Alveolar Surface Tension
Alveoli are lined by a thin fluid layer with high surface tension.
If unchecked, alveoli would collapse due to:
P=2T / r
(Law of Laplace)
Where:
P = collapsing pressure
T = surface tension
r = alveolar radius
Role of Pulmonary Surfactant
Produced by Type II pneumocytes, composed of:
-
Dipalmitylphosphatidylcholine (DPPC) – major component
-
Phosphatidylglycerol
-
Surfactant proteins (SP-A, SP-B, SP-C, SP-D)
Function:
-
Reduces alveolar surface tension
-
Prevents collapse (atelectasis)
-
Stabilizes alveoli of different sizes
-
Increases lung compliance
-
Essential for neonatal survival
Emulsification in Digestion
Surface tension is crucial in:
-
Fat emulsification by bile salts
-
Formation of micelles
-
Pancreatic lipase activity
Bile salts reduce surface tension, enabling large fat globules to break into micelles, improving digestion and absorption.
Laboratory Techniques Based on Surface Tension
1. Capillary Action
Used in:
-
Hematocrit estimation
-
Urinometer
-
ESR tubes
-
Microhematocrit capillaries
Capillarity is driven by surface tension differences between the liquid and capillary walls.
2. Electrophoresis
Protein migration is influenced by buffer surface tension, which affects:
-
Gel hydration
-
Pore size
-
Protein mobility
3. Chromatography
Surface tension affects:
-
Solvent spreading
-
Capillary flow
-
Separation efficiency
4. Spectrophotometry & Sample Handling
-
Air bubbles in cuvettes form due to surface tension → cause light scattering errors
-
Proper pipetting technique overcomes surface tension effects
Surfactants
Surfactants (surface-active agents) lower surface tension by accumulating at the liquid–air interface.
Types of Surfactants
-
Anionic – bile salts, SDS
-
Cationic – quaternary ammonium compounds
-
Non-ionic – Triton X-100, Tween-20
-
Zwitterionic – CHAPS
Biochemical Uses
-
Solubilizing membrane proteins
-
Cell lysis
-
Enzyme assays
-
SDS-PAGE for protein separation
-
Emulsification and stabilization
Factors Influencing
| Factor | Effect | Biochemical Relevance |
|---|---|---|
| Temperature | ↓ ST | Pulmonary compliance ↑ |
| pH | Affects ionization of surfactants | Affects enzyme assays |
| Electrolytes | Change cohesive forces | Protein solubility |
| Organic solvents | Reduce ST | Used in lipid extraction |
| Presence of lipids | Reduce ST | Bile salts, lung surfactant |
Measurement of Surface Tension in Laboratories
1. Du Noüy Ring Method
-
Measures the force required to detach a ring from the surface
-
Common in biochemistry labs
2. Wilhelmy Plate Method
-
Measures the force acting on a thin plate
-
Highly accurate
3. Drop Volume/Drop Weight Methods
-
Used in physical chemistry labs
-
Relate drop size to surface tension
4. Capillary Rise Method
-
Based on the height of the liquid rise in a capillary
-
Useful for teaching labs
Significance in Biochemistry
Biological Membranes:
-
- Cell Membranes: The fluid-mosaic model of cell membranes illustrates how surface tension helps maintain the integrity of the lipid bilayer.
- Surface tension impacts how proteins and lipids interact at the membrane interface, affecting cellular functions such as signal transduction and membrane fluidity.
- Pulmonary Surfactant: In the lungs, surfactant reduces surface tension in the alveoli (air sacs) to prevent their collapse during exhalation.
- This is crucial for efficient gas exchange and respiratory function.
Protein Folding:
-
- Hydrophobic Interactions: Surface tension influences the folding and stability of proteins.
- Hydrophobic regions of proteins tend to cluster away from the aqueous environment, driven by surface tension and reducing the system’s overall free energy.
Enzyme Function:
-
- Substrate Binding: Surface tension affects how enzymes interact with substrates, especially in aqueous environments.
- The interaction between enzymes and substrates can be influenced by the surface tension of the solution, impacting reaction rates and efficiency.
Formation of Biological Structures:
-
- Micelles and Liposomes: Surface tension forms micelles and liposomes, which are essential for various biochemical processes, including drug delivery and cellular transport.
- These structures form due to the self-assembly of surfactants in aqueous environments, driven by minimizing surface tension.
Drug Delivery Systems:
-
- Nanoparticles and Vesicles: In pharmaceutical applications, surface tension affects the stability and release of drug-loaded nanoparticles and vesicles.
- Controlling surface tension helps in designing effective delivery systems with optimal release profiles.
Biochemical Assays:
-
- Drop Shape Analysis: Surface tension is used in various biochemical assays to study interactions at interfaces, such as the adsorption of proteins or lipids to surfaces, which can provide insights into molecular interactions and surface properties.
Cellular Mechanics:
-
- Cell Shape and Movement: Surface tension influences cell shape and movement.
- For instance, the tension at the cell surface affects processes like cell division, migration, and the formation of cellular structures.
MCQs
1. Surface tension is defined as:
a) Force per unit volume
b) Force per unit length
c) Pressure per unit area
d) Energy per unit mass
2. SI unit of surface tension is:
a) N/m
b) N/m²
c) Joule
d) Pascal
3. Surface tension is caused mainly due to:
a) Adhesive forces
b) Cohesive forces
c) Friction
d) Gravitational force
4. Symbol used for surface tension is:
a) α
b) γ
c) β
d) θ
5. Surface tension decreases with:
a) Increase in temperature
b) Decrease in temperature
c) Adding salt
d) Increasing pressure
6. Which decreases surface tension of water?
a) Common salt
b) Sugar
c) Soap or detergent
d) Ice
7. Surface tension is maximum in:
a) Hot water
b) Cold water
c) Water at 100°C
d) Water at critical temperature
8. Surface tension is minimum at:
a) Melting point
b) Room temperature
c) Critical temperature
d) Freezing point
9. Dimensions of surface tension are:
a) [M L T⁻²]
b) [M T⁻²]
c) [L T⁻¹]
d) [M L² T⁻²]
10. Capillary rise occurs due to:
a) Surface tension
b) Osmosis
c) Viscosity
d) Diffusion
11. Water forms spherical droplets because:
a) Atmospheric pressure
b) Surface tension minimizes surface area
c) Gravity
d) Adhesive force
12. Surface energy is equal to:
a) Surface tension × area
b) Mass × surface tension
c) Surface tension / area
d) Volume × surface tension
13. Excess pressure inside a soap bubble is:
a) 2γ/r
b) γ/r
c) 4γ/r
d) 3γ/r
14. Which liquid has the highest surface tension at room temperature?
a) Ethanol
b) Acetone
c) Water
d) Mercury
15. Which of the following increases surface tension of water?
a) Detergent
b) Soap
c) Heating
d) Dissolved salts
16. Interfacial tension refers to tension between:
a) Solid and gas
b) Two immiscible liquids
c) Liquid and solid
d) Gas and vacuum
17. The force due to surface tension on a wire frame is:
a) F = γl
b) F = 2γl
c) F = γl²
d) F = γ/l
18. Which method is used to measure surface tension?
a) Vernier method
b) Capillary rise method
c) Screw gauge method
d) Sonometer
19. A detergent acts as:
a) Surface tension increaser
b) Surface tension reducer
c) Viscosity increaser
d) Solubility reducer
20. Surface tension of a liquid becomes zero at:
a) Melting point
b) Freezing point
c) Boiling point
d) Critical temperature
✅ ANSWERS
-
b) Force per unit length
-
a) N/m
-
b) Cohesive forces
-
b) γ
-
a) Increase in temperature
-
c) Soap or detergent
-
b) Cold water
-
c) Critical temperature
-
b) [M T⁻²]
-
a) Surface tension
-
b) Surface tension minimizes surface area
-
a) Surface tension × area
-
c) 4γ/r
-
d) Mercury
-
d) Dissolved salts
-
b) Two immiscible liquids
-
b) 2γl
-
b) Capillary rise method
-
b) Surface tension reducer
-
d) Critical temperature
