- In histopathology, certain tissues require specialized treatment due to their unique composition or sensitivity to degradation.
- These tissues include the eyeball, bone marrow biopsies, and calcified bones.
- Each tissue poses unique challenges in fixation, decalcification, sectioning, and staining, requiring specific methods to preserve their structures and reveal diagnostic details.
- Below is a detailed analysis of these tissue types and the specialized techniques used in histopathology.
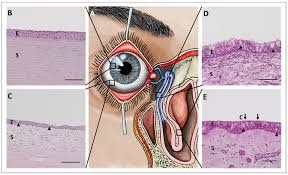
Eyeball (Ocular Tissue) in Histopathology
- The eye’s anatomy is complex, comprising delicate, layered tissues such as the cornea, lens, retina, and optic nerve, each with specific functions essential for vision.
- Preserving these histopathological structures is crucial for diagnosing ocular diseases like retinoblastoma, uveal melanoma, and glaucoma.
Fixation for Ocular Tissues
- Challenges: The eye’s unique structure makes it susceptible to rapid autolysis, particularly in the retina. The vitreous humor, a gel-like structure within the eye, also complicates fixation because it can impede the penetration of fixatives.
- Special Fixatives:
- Davidson’s Fixative: This mixture contains formalin, alcohol, and glacial acetic acid, which reduces tissue shrinkage and distortion, making it suitable for ocular tissues.
- Modified Bouin’s Solution: Though less common, it contains picric acid, which can help fix delicate ocular tissues.
- Technique:
- Whole globes are often left intact for fixation to prevent collapse and distortion of the internal structures.
- Small incisions at the scleral level can improve fixative penetration without damaging the eye’s architecture.
Sectioning and Grossing for Ocular Tissues
- Grossing: Standard sections for ocular tissue include anterior-posterior sections to capture the cornea, lens, retina, and optic nerve head.
- Transverse and Sagittal Sections capture specific areas like the macula and optic disc.
- Embedding Orientation: Ensuring correct orientation during embedding is critical to accurately analyze layers such as the choroid, retina, and sclera in histological sections.
- Section Thickness: Thin sections (typically 4–6 µm) allow for better resolution of small structures such as retinal layers.
Decalcification in Cases with Calcification
- Mild decalcification is performed for ocular tissues with calcified areas (e.g., scleral plaques).
- EDTA Decalcification: Preferred for ocular tissues because it minimizes the destruction of delicate structures.
- End-Point Testing: Radiographic methods or chemical tests (e.g., ammonium oxalate test) monitor decalcification to avoid over-softening.
Staining Techniques for Ocular Tissues
- Standard H&E Stain: Provides essential information on cell morphology.
- Specialized Stains:
- Periodic Acid-Schiff (PAS): Highlights basement membranes, particularly useful in the retinal pigment epithelium and Bruch’s membrane.
- Masson’s Trichrome: Assists in visualizing collagen, providing insights into any fibrosis or scarring.
- Reticulin Stain: Useful for demonstrating reticular fibers, often in the sclera.
- Immunohistochemistry: Antibodies against specific markers (e.g., GFAP for glial cells, melan-A for uveal melanomas) can help identify particular cell types or pathologies.
Bone Marrow Biopsy in Histopathology
- Bone marrow biopsies are crucial for diagnosing and monitoring hematological conditions.
- Special handling is required because bone marrow comprises soft and hard tissue elements.
Fixation for Bone Marrow Biopsies
- Challenges: Cellular elements in bone marrow are prone to autolysis. Fixation must, therefore, be prompt to preserve the bone marrow’s architecture and cellularity.
- Preferred Fixatives:
- 10% Neutral Buffered Formalin: Most commonly used fixative for bone marrow. Fixation should last 12–24 hours for adequate tissue penetration.
- B-5 Fixative: Provides excellent cellular detail but is less commonly used due to mercury content, which poses health and environmental hazards.
Decalcification of Bone Marrow Biopsies
- Importance of Decalcification: Trabecular bone in the core biopsy requires decalcification to allow for thin sectioning without damaging cellular structures.
- Agents:
- Formic Acid: Rapid decalcification, often completed within a few hours, may affect nucleic acid preservation if used for extended periods.
- EDTA is preferred for cases where molecular analyses are anticipated, as it is a gentler decalcifier that preserves DNA/RNA.
- End-Point Testing: After decalcification, radiography or testing the softness of the bone can confirm if decalcification is complete.
Processing and Embedding
- Orientation and Sectioning:
- Longitudinal orientation is essential for assessing cellularity, architecture, and overall biopsy quality.
- Section Thickness: Typically, sections are cut at 3–5 µm for optimal visualization.
Staining Techniques for Bone Marrow
- H&E Stain: Provides an initial overview of bone marrow architecture.
- Special Stains:
- Giemsa is particularly useful for visualizing fine nuclear detail and assessing cellular differentiation.
- Reticulin Stain: Helps in the evaluation of marrow fibrosis.
- Iron Stains: Essential in diagnosing conditions with iron overload or deficiency (e.g., Prussian blue for iron storage).
- Immunohistochemistry: CD markers (e.g., CD34 for progenitor cells, CD20 for B-cells, and CD138 for plasma cells) aid in identifying different hematopoietic and lymphoid lineages.
- Molecular Studies: Decalcified bone marrow biopsies can still undergo molecular analyses, such as PCR or FISH, provided EDTA is used for decalcification.
Calcified Bones in Histopathology
- Calcified bones are critical for diagnosing bone pathologies, including metabolic bone disease, osteosarcoma, and osteomyelitis.
- Calcified tissues pose unique challenges for histopathologists, particularly due to the hardness of calcium deposits, which require specialized processing.
Fixation for Calcified Bone
- Preferred Fixative: 10% neutral buffered formalin is commonly used, providing good fixation without excessive shrinkage or interference with subsequent decalcification.
- Alternative Fixatives:
- Ethanol-based solutions may be used in cases where mineral analysis is required, as they do not dissolve calcium deposits.
Decalcification of Calcified Bone
- Decalcification Agents:
- 10% Formic Acid: Effective for rapid decalcification, generally used for routine histopathological examination.
- EDTA: Preserves DNA and protein structure better than acids, making it ideal for cases needing molecular studies. However, decalcification with EDTA is slower.
- Microwave-Assisted Decalcification: This technique shortens decalcification time by applying controlled heat, though careful monitoring is necessary to prevent tissue damage.
- End-Point Testing: Radiographic imaging or simple bending tests help verify if decalcification is complete.
Processing, Embedding, and Sectioning
- After decalcification, bones are dehydrated, cleared, and embedded in paraffin.
- Section Thickness: Standard thickness is 4–6 µm, but undecalcified bone sections may require specialized microtomy techniques.
Staining Techniques for Calcified Bone
- H&E Stain: The primary stain highlights bone matrix and marrow components.
- Special Stains for Bone:
- Masson’s Trichrome: Differentiates between collagen (blue) and muscle/red blood cells, useful in examining bone matrix.
- Von Kossa Stain: Specifically highlights calcium deposits, aiding in the evaluation of mineralization.
- Alizarin Red Stain: Stains calcium deposits red, commonly used in metabolic bone disease research.
- Immunohistochemistry: Markers for osteoblasts (osteocalcin), osteoclasts (TRAP), and specific bone matrix proteins help identify bone-specific cell types and structural proteins.
- Fluorescent Labeling and Microscopy: In undecalcified sections, fluorochrome labeling can assess bone growth rates by analyzing mineral deposition patterns.