
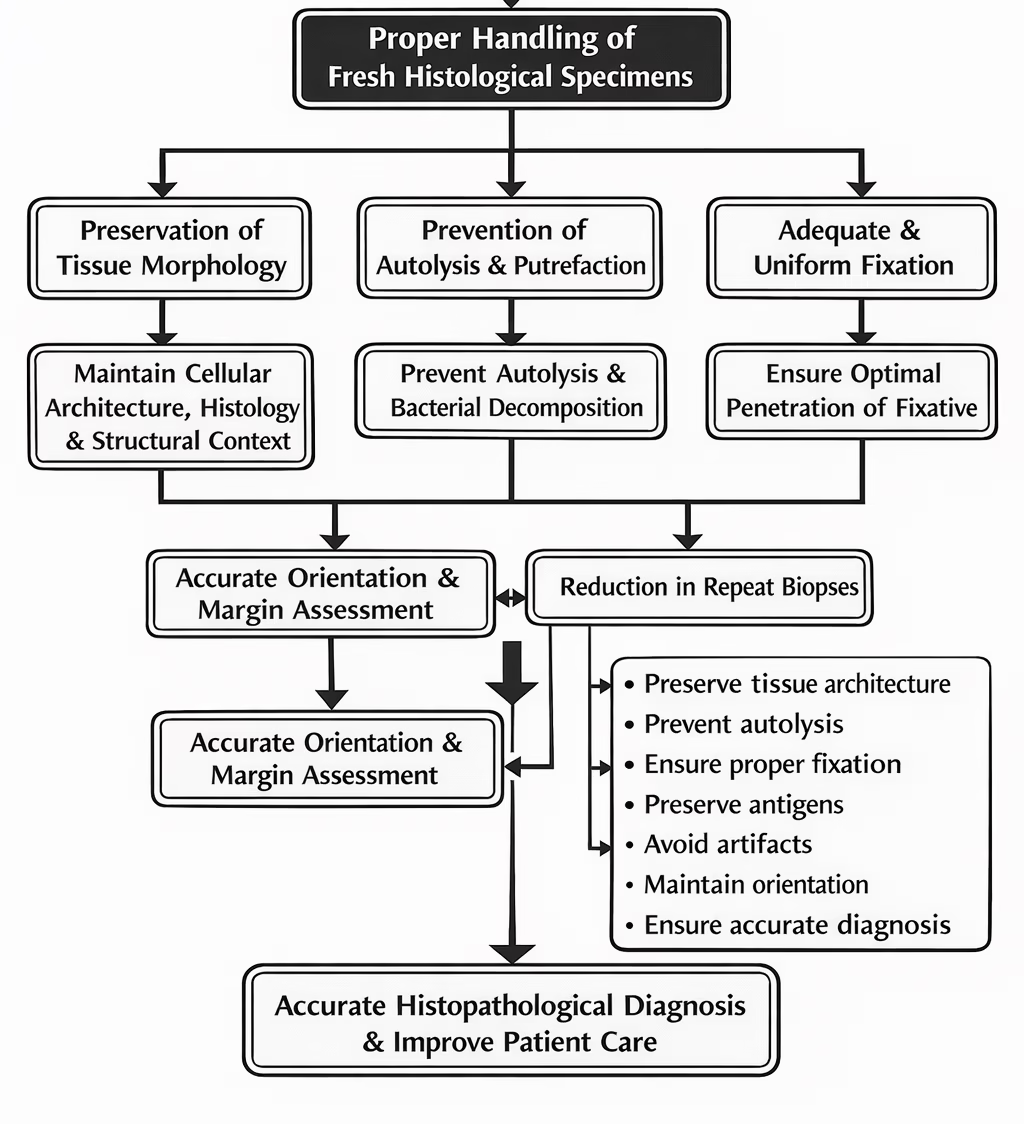
Introduction
-
Handling of fresh histological specimens is the first and most critical step in the histopathology workflow.
-
The quality of microscopic interpretation largely depends on how well the tissue is preserved before processing begins.
-
Improper handling can lead to autolysis, resulting in enzymatic degradation of tissues.
-
Inadequate handling may cause distortion of cellular and tissue architecture, affecting morphological assessment.
-
Poor specimen handling can result in loss of antigenicity, compromising immunohistochemistry and special staining techniques.
-
Errors during handling may lead to diagnostic inaccuracies or misinterpretation.
-
Since pre-analytical errors are irreversible, careful handling at this stage is essential.
-
Therefore, strict adherence to standardized specimen handling protocols is necessary to ensure accurate histopathological diagnosis and optimal patient care.

Objectives of Proper Handling of Fresh Specimens
-
To preserve native tissue morphology
-
To prevent autolysis and putrefaction
-
To ensure uniform fixation
-
To maintain cellular and subcellular details
-
To preserve antigens and nucleic acids for special studies
-
To avoid artifacts that interfere with diagnosis
Pre-Analytical Phase in Histopathology
Handling of fresh specimens forms part of the pre-analytical phase, which includes:
1. Specimen Collection
-
Tissue obtained by biopsy or surgical excision
-
Use of sharp instruments to avoid crush artifacts
-
Avoid excessive cautery near diagnostic margins
-
Immediate attention required after removal
2. Specimen Identification and Labeling
-
Correct labeling is mandatory and legally critical
-
Label must include:
-
Patient name and ID
-
Specimen type and site
-
Date and time of collection
-
-
Errors can lead to wrong diagnosis or wrong patient reporting
3. Clinical Information and Requisition Form
-
Proper clinical details help in accurate interpretation
-
Must include:
-
Clinical history
-
Provisional diagnosis
-
Type of procedure
-
Special requests (IHC, frozen section, molecular studies)
-
4. Transportation of Specimen
-
Transport should be rapid and safe
-
Avoid:
-
Drying
-
Excessive heat
-
Mechanical trauma
-
-
Delayed transport causes autolysis
5. Fixation
-
Most important step in the pre-analytical phase
-
Purpose:
-
Prevent autolysis and putrefaction
-
Preserve morphology
-
-
10% Neutral Buffered Formalin is the fixative of choice
-
Fixative-to-tissue ratio: 10 : 1
6. Gross Examination (Grossing)
-
Includes:
-
Inspection
-
Measurement
-
Orientation
-
Sectioning
-
-
Large specimens must be:
-
Opened
-
Sliced (≤5 mm thickness)
-
Margins identified and inked if required
-
7. Specimen-Specific Handling
-
Small biopsies: prevent loss using biopsy paper
-
Large specimens: ensure proper slicing
-
Frozen section samples: sent fresh without fixative
-
Molecular studies: special handling required
Collection of Fresh Histological Specimens
General Principles of Collection
-
Handle tissues gently and minimally
-
Use sharp, clean instruments
-
Avoid squeezing or crushing the specimen
-
Minimize the time between removal and fixation
-
Never allow tissue to dry out
-
Ensure proper orientation when required
Instruments Used for Collection
-
Scalpel with sharp blades
-
Fine tissue forceps (preferably toothed)
-
Scissors
-
Biopsy needles (for core biopsies)
-
Punch biopsy instruments
Excessive pressure with forceps should be avoided to prevent crush artifacts.
Methods of Specimen Collection
1. Biopsy Specimens
-
Small tissue samples obtained for diagnostic purposes
-
Types include:
-
Incisional biopsy
-
Excisional biopsy
-
Punch biopsy
-
Needle biopsy
-
-
Specimen should be immediately placed in fixative
2. Surgical Resection Specimens
-
Larger specimens obtained during operative procedures
-
Should be handled carefully to preserve anatomical relationships
-
Margins may require identification using ink or sutures
3. Endoscopic Biopsy Specimens
-
Very small and delicate
-
Prone to curling and drying
-
Should be placed on biopsy paper or sponge before fixation
4. Frozen Section Specimens
-
Collected fresh without fixative
-
Sent immediately to laboratory
-
Used for intraoperative diagnosis
Handling During Collection
-
Avoid contact with:
-
Disinfectants
-
Saline for prolonged periods
-
-
Blood clots should not be forcibly removed
-
Large specimens should not be tightly packed
Size and Thickness of Specimens
-
Thick tissues (>5 mm) prevent proper fixative penetration
-
Large specimens should be sliced immediately
-
Thin slices allow uniform fixation
Container Selection
-
Use wide-mouth, leak-proof containers
-
Container size should accommodate specimen without compression
-
Fixative volume should be 10 times the tissue volume
Immediate Post-Collection Steps
-
Place specimen in fixative without delay
-
Ensure correct labeling
-
Complete requisition form with clinical details
-
Transport promptly to laboratory
Common Errors During Collection
-
Delay in fixation
-
Crushing with forceps
-
Use of blunt instruments
-
Improper specimen orientation
-
Insufficient tissue volume
-
Allowing tissue to dry
Identification, Labeling, and Documentation
Identification of Histological Specimens
Identification refers to confirming the origin of the specimen and linking it to the correct patient.
Key Identification Requirements
-
Patient’s full name
-
Unique identification number (hospital number / registration number)
-
Age and sex (where required)
-
Anatomical site of specimen
-
Laterality (right / left), if applicable
Specimen identification must be done at the time of collection, not later.
Labeling of Specimen Containers
Labeling is the physical marking of the specimen container with essential information.
Essential Labeling Information
Each specimen container must be labeled with:
-
Patient name
-
Patient identification number
-
Specimen type (biopsy, excision, resection)
-
Anatomical site
-
Date and time of collection
Labeling Guidelines
-
Labels should be clear, legible, and waterproof
-
Labels must be placed on the container body, not on the lid
-
Handwritten labels should be neat and indelible
-
One container = one specimen only
Documentation (Requisition Form)
Documentation refers to the histopathology request or requisition form accompanying the specimen.
Essential Information on Requisition Form
-
Patient details (name, age, sex, ID)
-
Clinical history and symptoms
-
Provisional or differential diagnosis
-
Type of specimen and procedure
-
Site and laterality
-
Date and time of collection
-
Name of clinician/surgeon
-
Special requests:
-
Immunohistochemistry
-
Frozen section
-
Molecular studies
-
Correlation Between Label and Requisition Form
-
Information on the container label must exactly match the requisition form
-
Any discrepancy must be resolved before processing
-
Unlabeled or mismatched specimens should not be processed until clarified
Role of Laboratory Personnel
-
Verify specimen identity on receipt
-
Cross-check label and requisition details
-
Record specimen entry in laboratory register or LIS
-
Communicate immediately if discrepancies are found
-
Maintain specimen traceability throughout processing
Legal and Ethical Importance
-
Specimens are legal medical documents
-
Mislabeling can result in:
-
Wrong diagnosis
-
Wrong treatment
-
Legal liability
-
-
Proper documentation ensures accountability and patient safety
Common Errors in Identification and Labeling
-
Incomplete labeling
-
Illegible handwriting
-
Labeling container lid instead of body
-
Mismatch between specimen and requisition form
-
Missing clinical details
Impact of Errors
-
Delay in diagnosis
-
Repeat biopsy procedures
-
Increased patient anxiety
-
Compromised patient care
-
Medicolegal issues
Transportation of Fresh Specimens
Containers Used for Transportation
-
Wide-mouth containers for large specimens
-
Small, secure containers for biopsies
-
Containers must be:
-
Clean
-
Unbreakable (preferably plastic)
-
Properly labeled
-
-
The container size should allow the specimen to lie freely without distortion
Transportation with Fixative
-
Most specimens are transported in 10% Neutral Buffered Formalin
-
Fixative volume should be at least 10 times the tissue volume
-
Large specimens should be opened or sliced before transport to allow fixative penetration
-
Ensure the container lid is tightly closed to prevent leakage
Transportation of Fresh (Unfixed) Specimens
Certain specimens must be transported fresh, without fixative, including:
-
Frozen section specimens
-
Tissues for enzyme histochemistry
-
Specimens for molecular studies
Key precautions:
-
Transport immediately to the laboratory
-
Keep specimen moist but not immersed in saline
-
Avoid drying
-
Maintain cold chain if required
Transportation Time
-
Delay in transportation leads to:
-
Autolysis
-
Loss of cellular detail
-
Poor staining quality
-
-
Ideally, transport should occur within minutes of specimen collection
-
For distant sites, adequate fixation before transport is mandatory
Temperature Considerations
-
Avoid excessive heat, which accelerates tissue degradation
-
Avoid freezing unless specifically indicated
-
Room temperature transport is acceptable for formalin-fixed specimens
Safety Precautions During Transport
-
Treat all specimens as potentially infectious
-
Use biohazard-labeled containers if required
-
Avoid spillage of fixatives such as formalin
-
Personnel should use appropriate personal protective equipment (PPE)
Fixation
Purpose of Fixation
Fixation is a crucial step in histopathology that aims to preserve tissues in a state as close to life as possible. The main purposes of fixation are:
-
Arrests enzymatic degradation
Fixation inactivates intracellular enzymes that cause autolysis, thereby preventing self-digestion of tissues. -
Prevents bacterial growth
By killing microorganisms, fixation inhibits putrefaction and decomposition of tissues. -
Stabilizes tissue proteins
Fixatives denature and cross-link proteins, making cellular components insoluble and resistant to further degradation. -
Preserves structural relationships
Fixation maintains the normal architecture of cells and tissues, ensuring that morphological relationships are retained for accurate microscopic examination.
Fixative of Choice
-
10% Neutral Buffered Formalin (NBF) is the standard fixative used in routine histopathology.
-
It provides good preservation of:
-
Tissue morphology
-
Nuclear and cytoplasmic details
-
Antigenicity for immunohistochemistry
-
-
Buffering prevents the formation of formalin pigment and maintains an optimal pH.
Fixative Volume
-
The ideal fixative-to-tissue ratio is 10 : 1.
-
Adequate volume ensures:
-
Proper penetration of fixative
-
Uniform preservation of tissue
-
-
Insufficient fixative results in:
-
Incomplete fixation
-
Autolysis in deeper tissue layers
-
Poor staining and diagnostic artifacts
-
Handling According to Specimen Type
Proper handling of histological specimens varies depending on the size, nature, and intended diagnostic procedure. Adapting handling techniques according to specimen type is essential to preserve morphology, prevent artifacts, and ensure diagnostic accuracy.
1. Small Biopsy Specimens
Small biopsy specimens are delicate and highly prone to damage, loss, or distortion.
Handling Guidelines:
-
Place the specimen immediately in fixative after removal to prevent autolysis.
-
Avoid curling, folding, or crushing, as this can distort tissue architecture.
-
Use biopsy pads, sponges, or filter paper inside cassettes to prevent tissue loss during processing.
-
Ensure the specimen is fully submerged in fixative.
-
Over-fixation should be avoided, especially when the specimen is intended for immunohistochemistry (IHC), as prolonged fixation may mask antigenic sites.
Common Risks if Improperly Handled:
-
Tissue loss during processing
-
Crush artifacts
-
Poor IHC staining
2. Large Surgical Specimens
Large specimens require careful handling to allow uniform fixation and accurate pathological assessment.
Handling Guidelines:
-
Inspect and orient the specimen immediately upon receipt.
-
Identify anatomical landmarks and relevant margins.
-
Open hollow organs such as intestine, stomach, or uterus to allow fixative penetration.
-
Slice thick specimens into sections of ≤ 5 mm thickness to ensure adequate fixation.
-
Use surgical ink to mark resection margins when margin assessment is required.
-
Place the specimen in a container with adequate fixative volume (10:1 ratio).
Common Risks if Improperly Handled:
-
Incomplete fixation
-
Autolysis of deeper tissues
-
Inaccurate margin evaluation
3. Specimens for Frozen Section
Frozen section specimens are used for rapid intraoperative diagnosis and require special handling.
Handling Guidelines:
-
Specimens must be sent fresh without any fixative.
-
Keep the tissue moist but not immersed in saline or water.
-
Wrap gently in saline-moistened gauze if needed.
-
Transport rapidly to the histopathology laboratory.
-
Clearly communicate the need for frozen section to laboratory staff.
Important Note:
-
Delay in transport leads to freezing artifacts, tissue drying, and compromised diagnostic accuracy.
Prevention of Common Handling Artifacts
Proper specimen handling is essential to prevent artifacts that may obscure histological details and compromise diagnosis.
-
Crush Artifact
Caused by forceful handling with forceps or instruments.
Prevention: Handle tissues gently, use fine forceps, and avoid squeezing or pinching the specimen. -
Autolysis
Results from delayed fixation, leading to enzymatic degradation of tissue.
Prevention: Ensure immediate fixation after specimen removal. -
Drying Artifact
Occurs when tissue is exposed to air, causing cellular distortion.
Prevention: Never leave tissue exposed; place directly into fixative or keep moist if fresh tissue is required. -
Formalin Pigment (Acid Formaldehyde Hematin)
Appears as dark brown deposits due to acidic formalin.
Prevention: Always use neutral buffered formalin. -
Poor Fixation
Leads to uneven staining and loss of cellular detail.
Prevention: Slice thick tissues adequately (≤5 mm) to allow proper fixative penetration.
Special Handling Requirements
Certain specimens require modified handling protocols depending on downstream investigations.
Specimens for Immunohistochemistry (IHC)
Preservation of antigenicity is critical for reliable IHC results.
-
Avoid prolonged fixation, which can mask antigenic sites
-
Maintain consistent fixation time for reproducibility
-
Use 10% Neutral Buffered Formalin only
-
Avoid unbuffered or alternative fixatives unless specifically indicated
Specimens for Molecular Studies
Molecular diagnostic techniques require intact DNA and RNA.
-
Specimens may require:
-
Fresh tissue
-
Snap freezing (e.g., liquid nitrogen)
-
Special transport media
-
-
Avoid formalin fixation if DNA/RNA analysis is planned, as formalin causes nucleic acid fragmentation
-
Coordinate closely with the laboratory before specimen collection
Infectious and Hazardous Specimens
All tissues should be considered potentially infectious.
-
Treat all specimens as biohazardous
-
Follow biosafety and universal precautions
-
Use appropriate personal protective equipment (PPE)
-
Transport specimens in leak-proof, securely sealed containers
-
Clearly label high-risk specimens
Role of Laboratory Technician
Laboratory staff play a central role in ensuring specimen integrity and patient safety.
-
Verify specimen labeling and requisition form details
-
Ensure adequacy and appropriateness of fixation
-
Communicate any discrepancies or deficiencies immediately
-
Maintain specimen traceability throughout processing
-
Ensure proper documentation and record keeping
-
Strictly follow standard operating procedures (SOPs)
Quality Control in Specimen Handling
Quality assurance measures help maintain consistency and diagnostic reliability.
-
Use standardized fixation protocols
-
Conduct periodic training of clinical and laboratory staff
-
Monitor and document fixation times
-
Record pre-analytical variables affecting specimen quality
-
Perform regular audits of rejected or compromised specimens
-
Implement corrective actions when recurring errors are identified
Clinical Impact of Improper Specimen Handling
Errors in specimen handling have direct consequences on patient care.
-
Misinterpretation of tissue morphology
-
False-negative or false-positive diagnoses
-
Compromised immunohistochemistry results
-
Requirement for repeat biopsy procedures
-
Delay in diagnosis and patient management
-
Increased healthcare costs and medicolegal risk
MCQs
1. Handling of fresh histological specimens belongs to which phase?
A. Analytical
B. Post-analytical
C. Pre-analytical
D. Reporting
Answer: C
2. The most critical factor affecting histopathological diagnosis is:
A. Staining
B. Microscopy
C. Specimen handling
D. Reporting format
Answer: C
3. Immediate fixation is required mainly to prevent:
A. Shrinkage
B. Autolysis
C. Pigment formation
D. Overstaining
Answer: B
4. Autolysis is caused by:
A. Bacterial toxins
B. Fixatives
C. Tissue enzymes
D. Dehydration
Answer: C
5. The fixative of choice in routine histopathology is:
A. Alcohol
B. Bouin’s fluid
C. 10% Neutral Buffered Formalin
D. Glutaraldehyde
Answer: C
6. Ideal fixative-to-tissue ratio is:
A. 1 : 1
B. 5 : 1
C. 10 : 1
D. 20 : 1
Answer: C
7. Insufficient fixative volume leads to:
A. Better staining
B. Faster processing
C. Incomplete fixation
D. Over-fixation
Answer: C
8. Thick surgical specimens should be sliced to a thickness of:
A. ≤1 mm
B. ≤3 mm
C. ≤5 mm
D. ≥10 mm
Answer: C
9. Crush artifact is caused by:
A. Delayed fixation
B. Forceful handling
C. Excess fixative
D. Freezing
Answer: B
10. Drying artifact occurs when tissue is:
A. Over-fixed
B. Frozen
C. Exposed to air
D. Inked
Answer: C
11. Formalin pigment is prevented by using:
A. Acid formalin
B. Alcohol
C. Buffered formalin
D. Saline
Answer: C
12. Small biopsy specimens should be:
A. Left to dry briefly
B. Placed immediately in fixative
C. Washed in saline
D. Frozen routinely
Answer: B
13. To prevent loss of small biopsy specimens, use:
A. Cotton
B. Tissue paper
C. Biopsy pads/sponges
D. Gauze
Answer: C
14. Over-fixation is especially problematic for:
A. Routine H&E
B. Frozen section
C. Immunohistochemistry
D. Gross examination
Answer: C
15. Hollow organs should be:
A. Left unopened
B. Filled with saline
C. Opened before fixation
D. Frozen immediately
Answer: C
16. Surgical margins are best identified by:
A. Sutures only
B. Formalin
C. Surgical ink
D. Alcohol
Answer: C
17. Frozen section specimens must be:
A. Fixed in formalin
B. Sent fresh
C. Dehydrated
D. Paraffin embedded
Answer: B
18. Frozen section tissue should be kept:
A. Completely dry
B. Immersed in saline
C. Moist but not immersed
D. In alcohol
Answer: C
19. Delay in frozen section transport leads to:
A. Better sectioning
B. Improved staining
C. Diagnostic inaccuracy
D. Increased antigenicity
Answer: C
20. Labeling of specimen containers should be done:
A. After fixation
B. In the laboratory
C. Immediately at collection
D. During reporting
Answer: C
21. Specimen label must include all EXCEPT:
A. Patient name
B. Site of specimen
C. Diagnosis
D. Date/time
Answer: C
22. Labels should be placed on:
A. Container lid
B. Container body
C. Requisition form
D. Cassette only
Answer: B
23. Requisition form should include:
A. Clinical history
B. Provisional diagnosis
C. Special requests
D. All of the above
Answer: D
24. Mismatch between label and form should be:
A. Ignored
B. Corrected later
C. Resolved immediately
D. Reported after diagnosis
Answer: C
25. Transportation delay mainly causes:
A. Shrinkage
B. Autolysis
C. Swelling
D. Pigmentation
Answer: B
26. Best container for specimen transport is:
A. Narrow-neck glass bottle
B. Open tray
C. Wide-mouth leak-proof container
D. Paper bag
Answer: C
27. Fresh specimens for molecular studies should:
A. Always be fixed in formalin
B. Be snap-frozen or fresh
C. Be dehydrated
D. Be over-fixed
Answer: B
28. Formalin should be avoided for:
A. Routine histology
B. Grossing
C. DNA/RNA analysis
D. Margin assessment
Answer: C
29. All histological specimens should be treated as:
A. Non-infectious
B. Sterile
C. Potentially infectious
D. Harmless
Answer: C
30. Universal precautions are required mainly for:
A. Chemical safety
B. Electrical safety
C. Biosafety
D. Fire safety
Answer: C
31. PPE stands for:
A. Primary Processing Equipment
B. Personal Protective Equipment
C. Pathology Processing Environment
D. Protective Processing Element
Answer: B
32. Role of lab personnel includes:
A. Ignoring discrepancies
B. Verifying labeling
C. Fixing after processing
D. Reporting clinically
Answer: B
33. Specimen traceability means:
A. Staining quality
B. Fixation time
C. Ability to track specimen at all stages
D. Slide labeling
Answer: C
34. SOP stands for:
A. Standard Operating Procedure
B. Special Organ Processing
C. Specimen Orientation Protocol
D. Standard Output Program
Answer: A
35. Quality control in specimen handling includes:
A. Random fixation
B. Standardized protocols
C. Ignoring rejected samples
D. No documentation
Answer: B
36. Monitoring fixation time helps prevent:
A. Tissue loss
B. Over- or under-fixation
C. Poor labeling
D. Transport delay
Answer: B
37. Audit of rejected specimens helps in:
A. Increasing workload
B. Identifying recurring errors
C. Reducing diagnosis
D. Ignoring SOPs
Answer: B
38. Poor specimen handling may cause:
A. Accurate diagnosis
B. False-positive results
C. Faster reporting
D. Better IHC
Answer: B
39. Repeat biopsy is often required due to:
A. Good fixation
B. Proper handling
C. Poor specimen quality
D. Adequate labeling
Answer: C
40. Immunohistochemistry is mainly affected by:
A. Transport container color
B. Fixation quality
C. Grossing knife
D. Slide thickness
Answer: B
41. Cold ischemia time refers to delay between:
A. Fixation and processing
B. Processing and staining
C. Tissue removal and fixation
D. Staining and reporting
Answer: C
42. Mechanical damage to tissue results in:
A. Autolysis
B. Crush artifact
C. Drying artifact
D. Formalin pigment
Answer: B
43. Best practice to avoid drying artifact is:
A. Air exposure
B. Immediate fixation
C. Washing in water
D. Heating
Answer: B
44. Fixation mainly stabilizes:
A. Lipids only
B. Carbohydrates
C. Proteins
D. Minerals
Answer: C
45. The goal of proper specimen handling is:
A. Faster processing
B. Better reporting style
C. Accurate diagnosis
D. Reduced workload
Answer: C
46. Fresh specimen handling errors are:
A. Easily correctable
B. Partially reversible
C. Irreversible
D. Insignificant
Answer: C
47. Which specimen needs fastest transport?
A. Formalin-fixed tissue
B. Frozen section specimen
C. Paraffin block
D. Mounted slide
Answer: B
48. Fixation prevents bacterial growth by:
A. Washing bacteria
B. Killing microorganisms
C. Diluting tissue
D. Cooling tissue
Answer: B
49. Proper orientation of the specimen is important for:
A. Color of stain
B. Margin assessment
C. Dehydration
D. Clearing
Answer: B
50. Ultimately, improper specimen handling leads to:
A. Improved patient care
B. Delayed and incorrect management
C. Faster diagnosis
D. Better prognosis
Answer: B