Introduction
- Megaloblastic anemia is a type of anemia characterized by the presence of abnormally large, immature red blood cells (megaloblasts) in the bone marrow.
- This condition is often caused by impaired DNA synthesis, which affects the development and division of red blood cells.
- The result is fewer, larger red blood cells that are less effective at transporting oxygen throughout the body.
Causes
The most common causes of megaloblastic anemia include deficiencies in:
- Vitamin B12 (cobalamin): Often due to poor diet, absorption issues (e.g., pernicious anemia), or gastrointestinal diseases that affect absorption.
- Folate (Vitamin B9): This can be due to dietary deficiencies, malabsorption, alcoholism, certain medications, or increased requirements (e.g., during pregnancy).
Other less common causes include:
- Certain medications that interfere with DNA synthesis (e.g., chemotherapy drugs).
- Genetic disorders affecting vitamin metabolism.
Symptoms
Symptoms of megaloblastic anemia may include:
- Fatigue and weakness
- Shortness of breath
- Pale or jaundiced skin
- Glossitis (inflammation of the tongue)
- Neurological symptoms (more common in B12 deficiency) include tingling or numbness in the hands and feet, balance problems, and memory loss.
Laboratory investigations of megaloblastic anemia
Complete Blood Count (CBC)
The CBC is the foundation of diagnosing anemia and gives an overview of several hematological parameters:
- Hemoglobin (Hb): Hemoglobin levels are generally low in megaloblastic anemia, often significantly so, due to the reduced number of functioning RBCs. The degree of anemia can vary depending on the duration and severity of the vitamin B12 or folate deficiency.
- Mean Corpuscular Volume (MCV): In megaloblastic anemia, MCV is typically elevated above 100 fL and often reaches levels as high as 110-130 fL. MCV can sometimes reach even higher levels in severe cases. The MCV alone, however, cannot differentiate megaloblastic anemia from other macrocytic anemias (e.g., liver disease-related macrocytosis).
- Red Blood Cell (RBC) Count: The RBC count is usually low due to ineffective erythropoiesis. The production of defective cells and their premature destruction results in fewer circulating red cells.
- Red Cell Distribution Width (RDW): RDW is often increased (>14.5%) in megaloblastic anemia due to anisocytosis. This reflects the coexistence of large megaloblasts with smaller cells resulting from any compensatory marrow activity or concurrent deficiencies (e.g., iron).
- White Blood Cell (WBC) and Platelet Counts: In severe megaloblastic anemia, pancytopenia (low WBC, RBC, and platelets) may occur. This is due to the shared deficiency in DNA synthesis across all cell lineages. Reducing WBCs often involves neutropenia, and thrombocytopenia is typically mild to moderate.
Peripheral Blood Smear
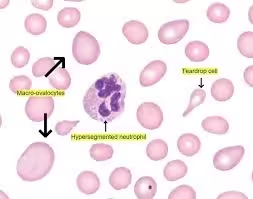
The peripheral blood smear in megaloblastic anemia provides significant clues by visualizing morphological abnormalities in blood cells:
- Macro-ovalocytes: Large, oval-shaped RBCs are distinctive for megaloblastic anemia. Macro-ovalocytes directly result from defective DNA synthesis, impairing nuclear maturation while the cytoplasm grows.
- Hypersegmented Neutrophils: Neutrophils with more than five nuclear lobes are common in megaloblastic anemia and are a specific finding. Hypersegmented neutrophils result from defective DNA synthesis, affecting granulocyte maturation.
- Anisopoikilocytosis: There is considerable variation in RBC shape (poikilocytosis) and size (anisocytosis), with a predominance of larger cells. This mixed morphology contributes to the increased RDW on CBC.
- Howell-Jolly Bodies: These nuclear remnants are due to impaired DNA synthesis. They can also be found in cases of splenectomy or functional asplenia but are common in severe megaloblastic anemia.
- Basophilic Stippling and Cabot Rings: Basophilic stippling and Cabot rings (nuclear remnants arranged in a ring) are occasionally observed, reflecting abnormal erythropoiesis.
Reticulocyte Count
The reticulocyte count is a marker of bone marrow activity:
- Low Reticulocyte Count: In megaloblastic anemia, the reticulocyte count is inappropriately low or normal, which indicates ineffective erythropoiesis. This contrasts with hemolytic anemia, where reticulocyte counts are typically elevated in response to increased RBC destruction. Reticulocytopenia results from prematurely destroying defective erythroid precursors within the bone marrow.
Bone Marrow Examination
A bone marrow examination may be necessary if the diagnosis is uncertain or if another cause of pancytopenia is suspected:
- Megaloblastic Changes: The bone marrow in megaloblastic anemia reveals megaloblasts, large erythroid precursors with delayed nuclear maturation. The nuclear chromatin remains open and immature while the cytoplasm matures, a feature called nuclear-cytoplasmic asynchrony.
- Giant Metamyelocytes and Band Forms: Large metamyelocytes and band forms with abnormal nuclear segmentation are observed in the granulocytic series. These findings indicate that DNA synthesis impairment affects all hematopoietic lineages.
- Hypercellularity: Bone marrow is typically hypercellular, reflecting an attempt to compensate for the anemia. However, this compensatory increase in cell production does not translate into an adequate reticulocyte response due to ineffective erythropoiesis.
- Decreased Myeloid/Erythroid Ratio: The erythroid precursors dominate in response to anemia, which lowers the myeloid/erythroid ratio compared to normal values (approximately 2:1 to 4:1).
Serum Vitamin B12 and Folate Levels
These tests are central to confirming the specific deficiency causing megaloblastic anemia:
- Vitamin B12: Low levels of serum vitamin B12 confirm deficiency. Values below 200 pg/mL typically indicate a deficiency, while levels between 200–300 pg/mL may be borderline, often prompting additional testing (e.g., MMA). B12 is necessary for DNA synthesis through its role in methylation and conversion of homocysteine to methionine.
- Folate: Both serum and RBC folate levels may be measured. Low serum folate levels confirm folate deficiency, but RBC folate levels offer a longer-term indication of folate stores, as serum levels can fluctuate with recent intake.
Methylmalonic Acid (MMA) and Homocysteine Levels
These metabolites help differentiate vitamin B12 and folate deficiencies:
- Methylmalonic Acid: MMA is an intermediate in fatty acid metabolism that requires vitamin B12 as a cofactor for conversion to succinyl-CoA. Elevated MMA levels (>0.4 µmol/L) are specific to vitamin B12 deficiency, making it useful in cases where vitamin B12 levels are borderline.
- Homocysteine: Homocysteine levels are elevated in vitamin B12 and folate deficiencies, as both vitamins are required for metabolism. Elevated homocysteine (>15 µmol/L) is a sensitive but non-specific marker, requiring further clarification with B12 and folate levels.
Intrinsic Factor Antibodies and Parietal Cell Antibodies
When vitamin B12 deficiency is confirmed, autoimmune testing helps determine if pernicious anemia is the underlying cause:
- Intrinsic Factor Antibodies (IF Ab): Intrinsic factor is crucial for vitamin B12 absorption, and intrinsic factor antibodies disrupt this process. Detection of these antibodies is highly specific for pernicious anemia, although sensitivity is moderate (~50–70%).
- Parietal Cell Antibodies (PCA): These antibodies target the parietal cells in the stomach that produce intrinsic factors. They are less specific (also found in other autoimmune diseases), but their presence supports a diagnosis of pernicious anemia when intrinsic factor antibodies are absent.
Lactate Dehydrogenase (LDH) and Bilirubin Levels
Increased cell turnover within the bone marrow results in the release of LDH and bilirubin:
- LDH: Elevated LDH (often 3-10 times the upper limit) is common due to intramedullary hemolysis of defective cells, reflecting increased turnover of blood cells.
- Bilirubin: Elevated levels of indirect (unconjugated) bilirubin are due to the breakdown of defective RBC precursors, although levels are typically not as high as in severe hemolytic anemias.
Iron Studies
Although iron metabolism is not directly involved, iron studies can help rule out concurrent iron deficiency:
- Serum Iron, Ferritin, and TIBC: Iron levels and storage are usually normal in megaloblastic anemia but may be low if there is concomitant iron deficiency.
Thyroid Function Tests (TFTs)
Hypothyroidism is known to cause mild macrocytosis and should be ruled out, especially in cases with unexplained anemia:
- Thyroxine (T4) and Thyroid-Stimulating Hormone (TSH): These hormones assess thyroid function. Elevated TSH with low T4 is diagnostic of hypothyroidism, which can contribute to macrocytosis and anemia in some cases.
Renal and Liver Function Tests
Renal and liver function tests rule out other causes of macrocytosis:
- Renal Function: Elevated blood urea nitrogen (BUN) or creatinine may suggest chronic kidney disease, a potential cause of anemia.
- Liver Function: Liver disease can cause macrocytosis due to altered lipid composition in RBC membranes.
Additional and Advanced Testing
If basic tests are inconclusive, further analyses may be done:
- DNA Testing for Genetic Disorders: In rare, resistant cases, DNA testing may be required to assess for congenital disorders of metabolism or genetic mutations associated with macrocytic anemias.
- Flow Cytometry for RBC Folate: This is occasionally employed to assess folate deficiency, providing a longer-term view of folate status.