Definition
Acid-base balance regulates the body’s hydrogen ion (H⁺) concentration to maintain the blood’s pH within the normal range of 7.35–7.45. Maintaining this balance is crucial for cellular function and overall homeostasis.
- The normal pH of arterial blood is 7.4
- pH of venous blood and interstitial fluids is about 7.35
- The blood pH is maintained within a remarkable constant level of 7.35 to 7.45.
Important of pH
- Changes in pH affect the ionization of protein molecules and, consequently activity of many enzymes.
- Changes in pH, together with the partial pressure of carbon dioxide (pCO2), change the shape of the hemoglobin.
- A decrease in pH increases sympathetic tone and may lead to cardiac dysrhythmias.
Metabolic sources of acids
During metabolic processes, two types of acids are produced
Fixed acids or non-volatile acids:
-
- Phosphoric
- Sulphuric acids
- Pyruvic acid,
- Lactic acid
- Keto acids
Volatile acids:
-
- Carbonic acid (H2CO3).
Metabolic sources of bases
- Citrate salts of fruit juices may produce bicarbonate salt.
- Deamination of amino acids produces ammonia
- The formation of bis-phosphate also contributes to the alkalinizing effect.
Maintenance of normal blood ph
To maintain the blood pH at 7.35 –7.45, three primary systems regulate the hydrogen ion concentration in the body fluids. These are:
- Buffer mechanism: First line of defense
- The respiratory mechanism: Second line of defense
- Renal mechanism: Third line of defense.
Buffer systems and their role in acid-base balance
- A buffer is a mixture of a weak acid and a salt of its conjugate base.
- A buffer can reversibly bind hydrogen ions. Free H+ combines with the buffer to form a weak acid (H buffer)
![]()
Blood Buffers
Various buffer systems present in the human body are
Buffers of extracellular fluid are present in plasma.
- Bicarbonate buffer
- Phosphate buffer
- Protein buffer
Buffers of intracellular fluid present in RBCs
- Bicarbonate buffer
- Phosphate buffer
- Hemoglobin buffer
Bicarbonate Buffer System (HCO3– / H2CO3)
- The bicarbonate buffer system is the most important extracellular buffer.
- It plays an important role in maintaining blood pH because of its high concentration.
- Two elements of the buffer system, bicarbonate (HCO3–) and carbonic acid (H2CO3), are regulated by the kidneys and by the lungs, respectively.
- Under physiological conditions, with a plasma pH 7.4, the bicarbonate to carbonic acid (HCO3– /H2CO3) ratio is 20:1.
Mechanism of action of bicarbonate buffer
When a strong acid, such as HCI, is added to the bicarbonate buffer solution, the increased hydrogen ions are buffered by HCO3– to form the very weak acid H2CO3, which, in turn, forms CO2 and H2O.
![]()
When sodium hydroxide (NaOH) is added to bicarbonate buffer, hydroxyl ion (OH–) from NaOH combines with H2CO3 to form weak base HCO3– and H2O
![]()
Phosphate Buffer System (HPO4– –/H2PO4–)
The phosphate buffer system is less important than a blood buffer; it plays a major role in buffering renal tubular fluid and intracellular fluids.
Mechanism of Action of Phosphate Buffer
When a strong acid such as HCI is added to phosphate buffer, the H+ is accepted by the base (hydrogen phosphate) HPO4– – and converted to (dihydrogen phosphate) H2PO4– and the strong acid HCI is replaced by a weak acid (sodium dihydrogen phosphate) NaH2PO4
![]()
When a strong base, such as NaOH, is added to the phosphate buffer, the OH– is buffered by the H2PO4– to form HPO4– – and water. Thus, strong base NaOH is replaced by weak base HPO4– –
![]()
At a plasma pH 7.4, the HPO4– – : H2PO4– is 4:1.
Protein Buffer (Na Protein/H Protein)
- In the blood, plasma proteins, especially albumin, act as a buffer.
- In acid solution, the basic amino group (NH2) takes up excess H+ ions forming (NH3+).
- In basic solutions, the acidic (carboxylic acids) COOH groups give up hydrogen ions forming (hydroxide) OH– of alkali to water.
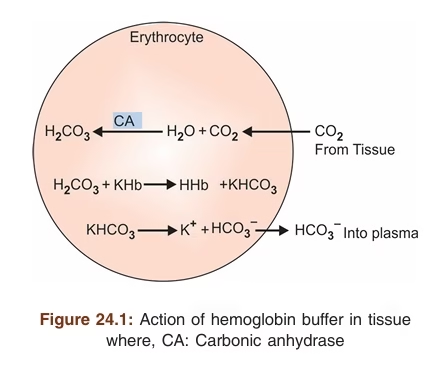
Hemoglobin Buffer
Hemoglobin is the major intracellular buffer of blood which is present in erythrocytes. It buffers carbonic acid (H2CO3) and anhydride CO2 from the tissues.
Action of hemoglobin buffer
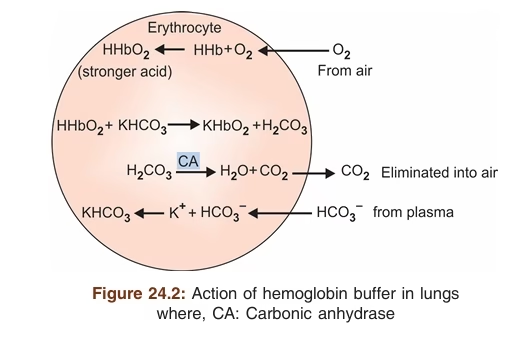
Respiratory Mechanism
- Second line of defense against acid-base disturbances
- It regulates the concentration of carbonic acid (H2CO3) in blood and other body fluids by the lungs.
- The respiratory center regulates the lungs’ removal or retention of CO2 and H2CO3 from the extracellular fluid.
- An increase in (H+) or (H2CO3) stimulates the respiratory center to increase the rate of respiratory ventilation, and excess acid (H2CO3) in the form of CO2 is quickly removed
- An increase in (OH–) or (HCO3–) depresses respiratory ventilation and release of CO2 from the blood
- The increased blood CO2 will result in the formation of more H2CO3 acid. To neutralize excess alkali (HCO3–)
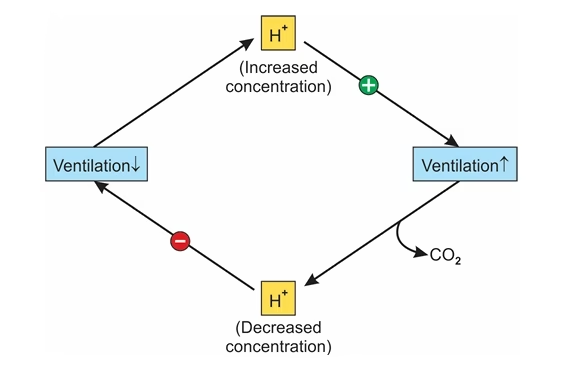
-
Acidosis (↑H⁺ / ↑H₂CO₃) → stimulates breathing → CO₂ removed → pH rises.
-
Alkalosis (↑OH⁻ / ↑HCO₃⁻) → depresses breathing → CO₂ retained → pH falls.
Renal mechanism in acid-base balance
- The renal mechanism is the third line of defense in acid-base balance. Renal mechanisms exert long-term acid-base control.
- The kidney regulates acid-base balance by conserving HCO3– (alkali reserve) and acid excretion.
- The pH of the initial glomerular filtrate is approximately 7.4, whereas the average urinary pH is approximately 6.0 due to the excretion of non-volatile acids produced by metabolic processes.
- The pH of the urine may vary from 4.5 to 8.0 corresponding to the case of acidosis or alkalosis.
- This ability to excrete variable amounts of acid or base makes the kidney the final defense mechanism against changes in body pH
Renal four key mechanisms
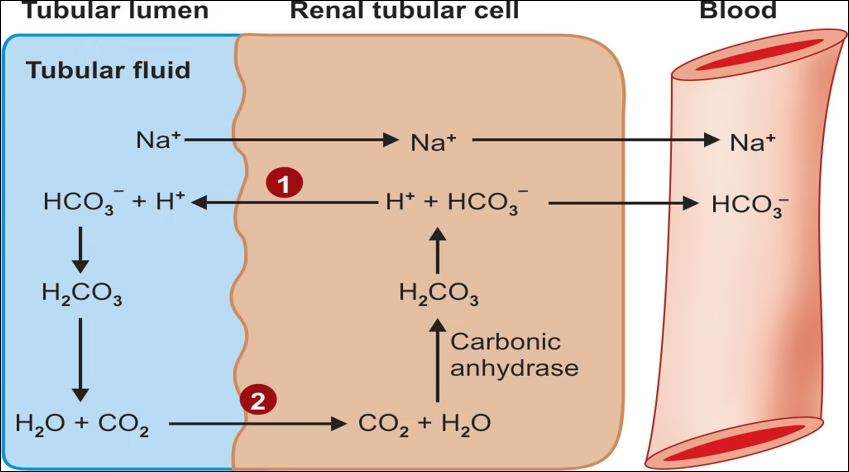
1.Exchange of H+ for Na+ of tubular fluid.
2. Reabsorption of bicarbonate from tubular fluid.
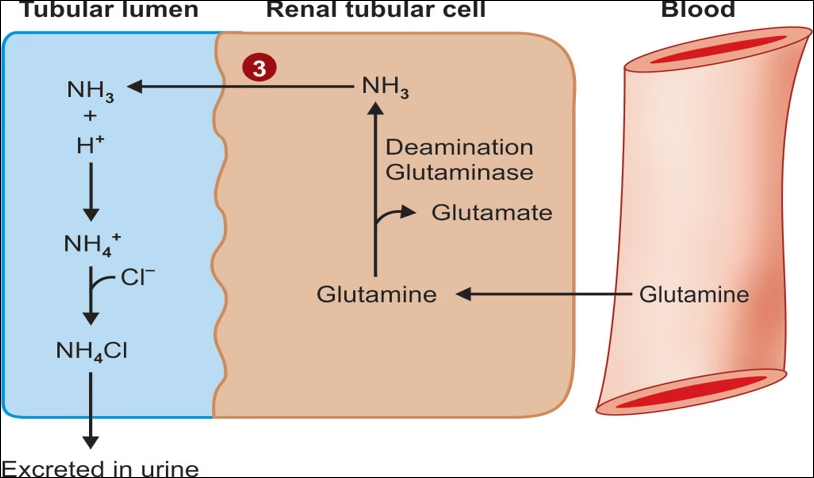
3. Formation of ammonia and excretion of ammonium ion (NH4+) in the urine.
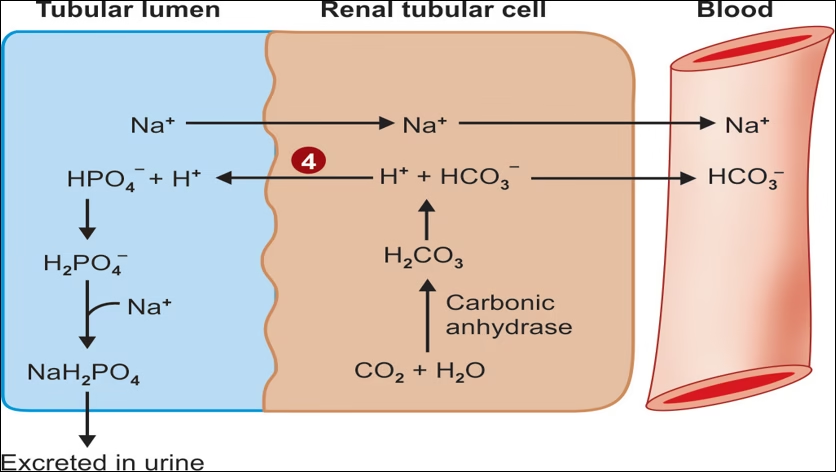
4.Excretion of H+ as (dihydrogen phosphate) H2PO4– in urine
Exchange of H+ for Na+ of tubular fluid and reabsorption of bicarbonate from tubular fluid.
Formation of ammonia and excretion of ammonium Ions in the urine.
Excretion of H+ as H2PO4 – in urine.
Disorders of Acid-Base Balance
- Acidemia is defined as an arterial blood pH of less than 7.35.
- Alkalemia is defined as an arterial blood pH of greater than 7.45.
- Acidosis and alkalosis refer to pathological states that can lead to acidemia or alkalemia.
Acidosis and alkalosis are classified in terms of their cause :
1. Metabolic Disorders (Problem in HCO₃⁻)
Metabolic Acidosis
-
Due to ↓ bicarbonate (HCO₃⁻)
-
Causes: DKA, diarrhea (loss of HCO₃⁻), renal failure, lactic acidosis.
Metabolic Alkalosis
-
Due to ↑ bicarbonate (HCO₃⁻)
-
Causes: vomiting (loss of H⁺), diuretics, excess antacids, hypokalemia.
2. Respiratory Disorders (Problem in CO₂ / H₂CO₃)
Respiratory Acidosis
-
Due to ↑ pCO₂ or ↑ H₂CO₃
-
Causes: COPD, hypoventilation, airway obstruction, CNS depression (opioids).
Respiratory Alkalosis
-
Due to ↓ pCO₂ or ↓ H₂CO₃
-
Causes: hyperventilation (anxiety, high altitude, fever, PE).
MCQs
-
Normal arterial blood pH range is:
A. 7.20–7.30
B. 7.30–7.40
C. 7.35–7.45
D. 7.45–7.55 -
The commonly quoted “normal” arterial pH (approx.) is:
A. 7.2
B. 7.35
C. 7.4
D. 7.5 -
Which of these is a volatile acid produced in metabolism?
A. Lactic acid
B. Phosphoric acid
C. Carbonic acid (H₂CO₃)
D. Sulfuric acid -
Which of these is classified as a fixed (non-volatile) acid?
A. Carbonic acid
B. Lactic acid
C. Carbon dioxide
D. Water -
The first line of defence against pH change in the body is:
A. Renal mechanism
B. Respiratory mechanism
C. Buffer systems
D. Bone buffering -
The second line of defence against acid–base disturbances is:
A. Buffer systems
B. Respiratory mechanism
C. Renal mechanism
D. Hepatic metabolism -
The third line (long-term) of defence against acid–base disturbances is:
A. Buffer systems
B. Respiratory mechanism
C. Renal mechanism
D. Hemoglobin buffering -
Which buffer system is the most important extracellular buffer?
A. Protein buffer
B. Phosphate buffer
C. Bicarbonate buffer (HCO₃⁻/H₂CO₃)
D. Hemoglobin buffer -
At physiological pH (~7.4) the bicarbonate:carbonic acid ratio (HCO₃⁻:H₂CO₃) is approximately:
A. 10:1
B. 20:1
C. 1:1
D. 50:1 -
The phosphate buffer ratio (HPO₄²⁻:H₂PO₄⁻) at plasma pH ~7.4 is approximately:
A. 1:4
B. 4:1
C. 1:1
D. 20:1 -
Which plasma protein is emphasized as an important protein buffer?
A. Globulin only
B. Albumin mainly
C. Hemoglobin only
D. Fibrinogen -
The hemoglobin buffer acts mainly within:
A. Plasma
B. Erythrocytes (RBCs) — intracellular fluid
C. Interstitial fluid
D. Bone -
Increased H⁺ (acidosis) stimulates which immediate response?
A. Decreased ventilation
B. Increased ventilation (respiratory compensation)
C. Increased renal HCO₃⁻ excretion
D. Increased bone mineralization -
Increased HCO₃⁻ or OH⁻ (alkalosis) causes which respiratory response?
A. Increased ventilation
B. Depressed ventilation (hypoventilation)
C. No change in ventilation
D. Immediate renal excretion of CO₂ -
The kidney helps long-term acid–base control by all EXCEPT:
A. Reabsorbing filtered bicarbonate
B. Excreting H⁺ as ammonium (NH₄⁺)
C. Excreting H⁺ as dihydrogen phosphate (H₂PO₄⁻)
D. Rapidly altering ventilation to remove CO₂ -
Approximate normal urinary pH range cited is:
A. 2.0–4.0
B. 4.5–8.0
C. 6.8–7.4 only
D. 8.5–10.0 -
Which of the following is not listed as a metabolic source of acids on the page?
A. Sulphuric acid
B. Phosphoric acid
C. Lactic acid
D. Bicarbonate -
Which of the following is listed as a metabolic source of bases?
A. Sulphuric acid
B. Citrate from fruit juices producing bicarbonate salts
C. Lactic acid
D. Keto acids -
Which buffer plays a major role in buffering renal tubular fluid and intracellular fluids?
A. Bicarbonate buffer
B. Phosphate buffer
C. Hemoglobin buffer
D. Protein buffer -
The bicarbonate buffer system components (HCO₃⁻ and H₂CO₃) are regulated mainly by:
A. Liver and pancreas
B. Kidneys (HCO₃⁻) and lungs (CO₂/H₂CO₃)
C. Intestine and spleen
D. Bone and skin -
When a strong acid (e.g., HCl) is added to the bicarbonate buffer, H⁺ is initially buffered by:
A. H₂O directly
B. HCO₃⁻ to form H₂CO₃ (which forms CO₂ + H₂O)
C. Protein buffers only
D. Phosphate buffer immediately -
When a strong base (e.g., NaOH) is added to bicarbonate buffer, OH⁻ combines with:
A. HCO₃⁻
B. H₂CO₃ to form HCO₃⁻ and water
C. Hemoglobin only
D. Albumin only -
The respiratory center regulates acid–base balance by controlling removal/retention of:
A. HCO₃⁻
B. CO₂ (and therefore H₂CO₃)
C. NH₄⁺
D. Phosphate -
Which renal mechanism is not one of the four key mechanisms listed?
A. Exchange of H⁺ for Na⁺ in tubular fluid
B. Reabsorption of bicarbonate from tubular fluid
C. Formation and excretion of ammonium (NH₄⁺)
D. Rapid conversion of CO₂ to HCO₃ in alveoli -
The kidney’s ability to vary urinary pH makes it the:
A. First line of defense
B. Final/long-term defense against pH change
C. Sole regulator of PaCO₂
D. Primary regulator of hemoglobin binding -
Acidemia is defined as arterial pH:
A. < 7.00
B. < 7.35
C. > 7.45
D. > 7.60 -
Alkalemia is defined as arterial pH:
A. < 7.35
B. > 7.45
C. = 7.40 only
D. < 7.20 -
Which buffer system has greatest capacity in extracellular fluid because of its high concentration?
A. Phosphate buffer
B. Protein buffer
C. Bicarbonate buffer
D. Hemoglobin buffer -
Hemoglobin buffer most directly buffers:
A. Bicarbonate in plasma
B. Carbonic acid and CO₂ from tissues within RBCs
C. Plasma albumin only
D. Renal tubular fluid exclusively -
The bicarbonate buffer’s effectiveness depends on which organ to remove CO₂ produced from H₂CO₃ decomposition?
A. Liver
B. Lungs (respiration)
C. Kidney only
D. Intestine -
Which of the following is TRUE about intracellular buffers listed?
A. Intracellular buffers include phosphate and hemoglobin only
B. Intracellular buffers include bicarbonate, phosphate and hemoglobin (in RBCs)
C. Only plasma has buffers; cells do not
D. Hemoglobin buffers only plasma, not intracellularly -
The article states that changes in pH affect the ionization of proteins and therefore:
A. Enzyme activity and protein function
B. Only hemoglobin and no other proteins
C. Only DNA replication rates
D. Only blood viscosity -
An increase in H⁺ (lower pH) will tend to cause what cardiovascular effect according to the article?
A. Bradycardia only
B. Increased sympathetic tone and potential dysrhythmias
C. Vasodilation without effect on heart
D. No cardiovascular effect -
Which statement about buffer action in the article is correct?
A. A buffer is a strong acid and its salt
B. A buffer is a mixture of a weak acid and its conjugate base (salt)
C. Buffers have no role in acid–base balance
D. Buffers only act in the kidney -
At plasma pH 7.4, which buffer ratio is highest (numerically) per the article?
A. HPO₄²⁻ : H₂PO₄⁻ (phosphate) = 4:1
B. HCO₃⁻ : H₂CO₃ (bicarbonate) = 20:1
C. Both are equal
D. Hemoglobin : albumin = 10:1 -
The bicarbonate buffer reacts to added HCl by forming:
A. H₂CO₃ (carbonic acid) which then yields CO₂ + H₂O
B. NaCl directly
C. NH₄Cl only
D. Phosphate salts -
The phosphate buffer is particularly important in buffering:
A. Plasma exclusively
B. Renal tubular fluid and intracellular fluids
C. Exhaled air
D. Bone matrix only -
Formation of NH₄⁺ by the kidney aids acid excretion because:
A. NH₄⁺ is a volatile base excreted in breath
B. Ammonia trapping allows excretion of H⁺ as ammonium in urine
C. NH₄⁺ increases plasma HCO₃⁻ directly within minutes
D. NH₄⁺ binds to hemoglobin for transport -
The pH of initial glomerular filtrate is approximately:
A. 5.0
B. 7.4
C. 6.0
D. 4.5 -
According to the article, which of these is not a buffer of extracellular fluid?
A. Bicarbonate
B. Phosphate
C. Protein
D. Hemoglobin -
Which buffer system acts quickly (seconds to minutes) to minimize pH change?
A. Renal mechanisms (hours to days)
B. Buffer systems (immediate)
C. Bone buffering only (days to weeks)
D. Cellular proliferation -
Which organ is responsible for regulating carbonic acid (H₂CO₃) concentration in blood?
A. Liver
B. Lungs (via CO₂ removal)
C. Kidney only
D. Pancreas -
Adding NaOH (strong base) to phosphate buffer will result in:
A. Conversion of H₂PO₄⁻ to HPO₄²⁻ and water (buffering)
B. Immediate increase in H₂PO₄⁻ only
C. Precipitation of phosphate crystals only
D. No reaction -
The bicarbonate buffer’s ratio 20:1 helps maintain pH; major controllers of the two components are:
A. Lungs control HCO₃⁻; kidney controls CO₂
B. Kidneys control HCO₃⁻; lungs control CO₂/H₂CO₃
C. Liver controls both
D. Intestine controls both -
Which of these is a direct consequence of decreased pH on hemoglobin?
A. No change in oxygen delivery
B. Change in hemoglobin shape affecting O₂ affinity (Bohr effect)
C. Hemoglobin becomes permanently denatured
D. Hemoglobin increases HCO₃⁻ synthesis -
The article lists which of the following among renal “four key mechanisms”?
A. Filtration of plasma proteins into urine
B. Exchange of H⁺ for Na⁺ of tubular fluid
C. Increasing secretion of insulin
D. Enhancing pulmonary ventilation -
Which buffer system has a significant role inside RBCs?
A. Albumin buffer
B. Hemoglobin buffer and intracellular phosphate/bicarbonate systems
C. Only plasma bicarbonate
D. Bone carbonate buffer -
How does the article describe a buffer chemically?
A. A strong acid and strong base pair
B. A mixture of a weak acid and a salt of its conjugate base
C. Pure water with high pH
D. A volatile gas -
Why is the kidney considered the final defense mechanism against body pH changes?
A. Because it can excrete variable amounts of acid or base over a wide pH range (4.5–8.0 urine pH)
B. Because it controls fast respiratory responses
C. Because it stores buffers long term in adipose tissue
D. Because it produces CO₂ directly -
Which of the following best summarizes the three primary systems that regulate hydrogen ion concentration?
A. Buffers, liver metabolism, skin excretion
B. Buffer mechanism (first), respiratory mechanism (second), renal mechanism (third)
C. Hemoglobin, bone, gut flora only
D. Pancreas, spleen, thyroid
Answer Key —
-
C
-
C
-
C
-
B
-
C
-
B
-
C
-
C
-
B
-
B
-
B
-
B
-
B
-
B
-
D
-
B
-
D
-
B
-
B
-
B
-
B
-
B
-
B
-
D
-
B
-
B
-
B
-
C
-
B
-
B
-
B
-
A
-
B
-
B
-
B
-
A
-
B
-
B
-
B
-
D
-
B
-
B
-
A
-
B
-
B
-
B
-
B
-
B
-
A
-
B