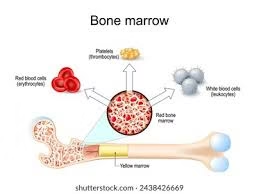
Hematopoiesis
- Hematopoiesis (from Greek: haima = blood, poiesis = formation) is the complex, lifelong process of blood cell production and development.
- It encompasses the proliferation, differentiation, maturation, and eventual release of all functional blood elements—erythrocytes (RBCs), leukocytes (WBCs), and thrombocytes (platelets)—from a pool of undifferentiated hematopoietic stem cells (HSCs).
This process ensures:
-
Maintenance of adequate oxygen transport via RBCs.
-
Defense against pathogens through WBCs.
-
Hemostasis and clot formation via platelets.
The balance of cell production and clearance is tightly regulated to respond to physiological needs, such as infection, bleeding, or hypoxia.
Physiological Aspects of Hematopoiesis
Sites of Hematopoiesis Throughout Life
Embryonic Development:
-
Mesoblastic phase (Weeks 2–8 gestation): The initial site is the yolk sac, where primitive nucleated erythroblasts are formed. This stage lacks definitive hematopoiesis and supports only early embryonic oxygen transport.
-
Hepatic phase (6 weeks–birth): Liver becomes the dominant site of blood cell production. Erythroid and myeloid precursors are now seen. The spleen and thymus also contribute.
-
Medullary (Myeloid) phase (after 5 months gestation): Bone marrow takes over as the primary site, with formation of all cell lineages in their mature forms.
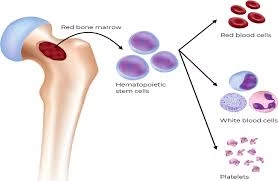
Postnatal and Adult Hematopoiesis:
-
At birth, almost all bones contain red marrow.
-
With age, hematopoiesis becomes restricted to axial skeleton (pelvis, sternum, ribs, vertebrae, skull).
-
Red marrow (hematopoietically active) progressively converts to yellow marrow (fatty and inactive), though yellow marrow can revert to red marrow under stress (e.g., chronic anemia, leukemia).
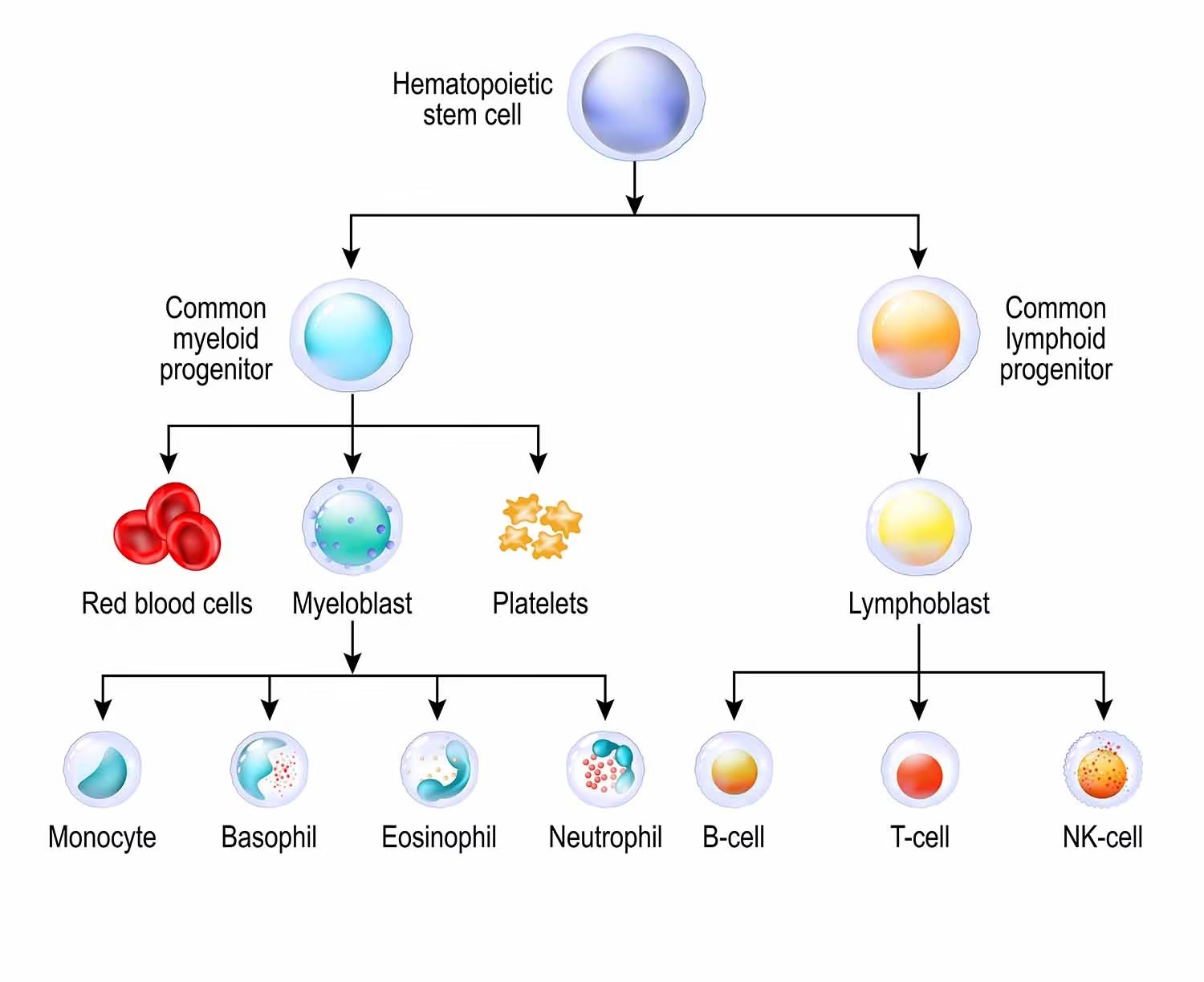
Hematopoietic Stem Cells (HSCs) and Progenitors
HSCs are multipotent, self-renewing cells that give rise to all mature blood cells.
-
Surface markers: CD34+, CD38−, Lin−.
-
Self-renewal: Maintains the stem cell pool.
-
Differentiation: Leads to formation of progenitor cells:
-
Common Myeloid Progenitor (CMP) → Erythrocytes, Megakaryocytes (→ Platelets), Granulocytes, Monocytes.
-
Common Lymphoid Progenitor (CLP) → B cells, T cells, Natural Killer (NK) cells.
-
Regulation of Hematopoiesis
Hematopoiesis is controlled by a combination of intrinsic transcriptional programming, extrinsic signaling from the microenvironment, and circulating growth factors.
A. Cytokines and Hematopoietic Growth Factors
| Growth Factor | Source | Function |
|---|---|---|
| Erythropoietin (EPO) | Kidney (peritubular interstitial cells) | Stimulates RBC production in response to hypoxia |
| Thrombopoietin (TPO) | Liver, kidney, marrow stromal cells | Stimulates platelet production via megakaryocytes |
| Granulocyte Colony-Stimulating Factor (G-CSF) | Endothelial cells, macrophages | Stimulates neutrophil production |
| GM-CSF (Granulocyte-Macrophage CSF) | Macrophages, T cells | Broad stimulation of granulocytes and monocytes |
| IL-3, IL-6, SCF (Stem Cell Factor) | Various sources | Promote survival, proliferation, and differentiation of multiple lineages |
B. Feedback Loops
-
Erythropoiesis is controlled by renal sensing of oxygen tension.
-
Infection triggers IL-1, IL-6 → drives myelopoiesis (neutrophil production).
-
Hemorrhage stimulates thrombopoiesis via elevated TPO.
C. Bone Marrow Microenvironment (Niche)
The hematopoietic niche comprises:
-
Stromal cells (fibroblasts, adipocytes, osteoblasts).
-
Extracellular matrix (ECM) proteins (fibronectin, collagen, laminin).
-
Adhesion molecules (VCAM-1, ICAM-1) that regulate stem cell retention.
-
Oxygen gradient: Low O₂ tension in the endosteal region helps maintain HSC quiescence.
Morphological Aspects of Hematopoiesis
Hematopoietic Cell Lineages
A. Erythropoiesis (7 days process)
-
Proerythroblast: Large nucleus, deeply basophilic cytoplasm.
-
Basophilic erythroblast: Nucleus begins to condense, intense RNA activity.
-
Polychromatic erythroblast: Cytoplasm becomes grayish as hemoglobin accumulates.
-
Orthochromatic erythroblast: Small, pyknotic nucleus; cytoplasm turns pinkish.
-
Reticulocyte: Nucleus extruded; residual RNA seen (reticular network).
-
Mature erythrocyte: Biconcave disc, no organelles, hemoglobin-rich.
B. Granulopoiesis
-
Myeloblast → Promyelocyte → Myelocyte → Metamyelocyte → Band form → Mature neutrophil/eosinophil/basophil.
-
Morphological changes include nuclear indentation, appearance of specific granules.
C. Monocytopoiesis
-
Monoblast → Promonocyte → Monocyte → Tissue macrophage (Kupffer cells, microglia, alveolar macrophages).
D. Lymphopoiesis
-
Lymphoid stem cell differentiates into:
-
B lymphocytes (mature in bone marrow).
-
T lymphocytes (migrate to thymus for maturation).
-
NK cells (cytotoxic lymphocytes).
-
E. Thrombopoiesis
-
Megakaryoblast → Promegakaryocyte → Megakaryocyte (giant, multilobed nucleus).
-
Platelets are formed by cytoplasmic fragmentation (approximately 1,000–3,000 per megakaryocyte).
Bone Marrow Architecture
-
Red marrow: Highly cellular with hematopoietic precursors.
-
Yellow marrow: Mostly adipocytes with sparse hematopoietic cells.
-
Sinusoids: Vascular channels allowing mature cells to enter circulation.
-
Reticulin framework: Supports developing cells and regulates their movement.
Biochemical Aspects of Hematopoiesis
Hemoglobin Synthesis
Occurs predominantly in erythroid precursors, involves:
A. Heme Synthesis (Mitochondrial & Cytoplasmic Steps)
-
δ-ALA Synthase (rate-limiting): Succinyl-CoA + Glycine → δ-Aminolevulinic acid (ALA).
-
ALA → Porphobilinogen → Uroporphyrinogen III → Coproporphyrinogen III.
-
Mitochondria: Coproporphyrinogen → Protoporphyrin IX → Heme (by ferrochelatase with Fe²⁺).
-
Inhibited by lead poisoning (ALA dehydratase, ferrochelatase inhibition).
B. Globin Chain Synthesis
-
Occurs in cytoplasm; governed by α and β-globin gene expression.
-
Disorders: Thalassemia (reduced/absent globin chain synthesis).
Cellular Metabolism in Developing Cells
Erythrocytes:
-
Lack mitochondria; depend entirely on anaerobic glycolysis for ATP.
-
Use hexose monophosphate shunt (pentose phosphate pathway) for NADPH → protects against oxidative stress via glutathione.
Neutrophils:
-
High rate of aerobic glycolysis and oxidative phosphorylation.
-
Use NADPH oxidase system to produce superoxide radicals (respiratory burst) to kill microbes.
Essential Vitamins and Trace Elements
| Nutrient | Role in Hematopoiesis | Deficiency Consequence |
|---|---|---|
| Iron | Central to heme synthesis | Microcytic anemia |
| Vitamin B12 & Folate | DNA synthesis & methylation | Megaloblastic anemia |
| Vitamin C | Enhances iron absorption | Scurvy, anemia |
| Copper | Iron mobilization (via ceruloplasmin) | Hypochromic anemia |
| Zinc | DNA replication, cell division | Impaired immunity, anemia |
Transcriptional Regulation of Hematopoiesis
-
GATA-1: Promotes erythroid and megakaryocytic differentiation.
-
PU.1: Essential for granulocyte/monocyte lineages.
-
NOTCH1: T-cell commitment.
-
IKAROS: Master regulator of lymphoid lineage development.
These transcription factors interact with epigenetic modulators (histone acetylases, methylases) to fine-tune lineage-specific gene expression.
Apoptosis and Cell Cycle Control
-
Homeostasis: Maintained by eliminating excess or abnormal cells via apoptosis.
-
p53: Senses DNA damage and activates apoptotic pathways.
-
Bcl-2 family: Regulates mitochondrial apoptosis.
-
Cyclins/CDKs: Ensure orderly progression through the cell cycle.
Clinical Correlates
-
Aplastic anemia: Pancytopenia due to bone marrow failure.
-
Leukemias: Clonal proliferation of immature cells; morphology, cytogenetics, and flow cytometry are used for diagnosis.
-
Myelodysplastic syndromes (MDS): Ineffective hematopoiesis with dysplasia.
-
Bone Marrow Transplantation (BMT): Curative in many hematologic malignancies and genetic disorders.
Recent Advances
-
Single-cell transcriptomics: Mapping of lineage trajectories and intermediate states.
-
Gene therapy: Lentiviral and CRISPR-Cas9-based correction of β-thalassemia and sickle cell disease.
-
iPSC-derived hematopoiesis: Modeling diseases and regenerative therapies.
-
Artificial bone marrow scaffolds: Biomaterials designed to mimic marrow niche for ex vivo HSC expansion.