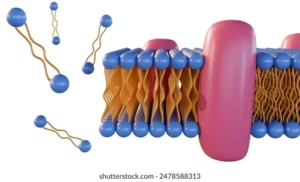
Definition
Lipids are a heterogeneous group of organic compounds that are insoluble in water but soluble in nonpolar solvents (like chloroform, ether, benzene). They are composed mainly of carbon, hydrogen, and a small amount of oxygen, and sometimes phosphorus, nitrogen, or sulfur.
👉 In chemistry, lipids include fats, oils, waxes, phospholipids, glycolipids, steroids, and related compounds
Properties of Lipids
- Lipids may be either liquids or non-crystalline solids at room temperature.
- Pure fats and oils are colourless, odourless, and tasteless.
- They are energy-rich organic molecules.
- Insoluble in water but in organic solvents like alcohol, chloroform, acetone, benzene, etc.
- No ionic charges.
Biological Importance of Lipids
- More palatable food
- Storable in unlimited amounts compared to carbohydrates.
- High-energy value
- Supply Essential fatty acids.
- Supply fat-soluble vitamins (A, D, E and K).
- Cholesterol – a. In membrane structure,
- Synthesis of some hormones,
- Synthesis of vitamin D3 and bile acids.
Classification of Lipids

Bloor’s (1943) Classification
Simple Lipids
They are esters of fatty acids with alcohol
They are divided into two groups –
-
Fats and Oils (True Fats / Neutral Fats)
-
Esters of fatty acids + glycerol.
-
Solid at room temperature = fats (mainly in animals).
-
Liquid at room temperature = oils (mainly in plants).
-
Function: energy storage, insulation, protection.
-
-
Waxes
-
Esters of fatty acids + long-chain alcohols (not glycerol).
-
Found on plant leaves, skin, and feathers.
-
Function: waterproofing and protection.
-
Compound lipids
Fatty Acid + Alcohol (Glycerol/Sphingosine) + additional groups
They are divided into 4 groups –
-
Phospholipids
-
Structure: Glycerol (or sphingosine) + 2 fatty acids + phosphate group + nitrogenous base.
-
Amphipathic (hydrophilic head + hydrophobic tails).
-
Types:
-
Glycerophospholipids (phosphoglycerides) – contain glycerol.
-
Examples: Lecithin (phosphatidylcholine), Cephalin (phosphatidylethanolamine).
-
-
Sphingophospholipids – contain sphingosine.
-
Example: Sphingomyelin (important in myelin sheath of nerves).
-
-
-
Functions:
-
Structural role: essential in cell membranes (lipid bilayer).
-
Cell signaling: involved in signal transduction and membrane fluidity.
-
2. Glycolipids
-
Structure: Sphingosine + fatty acid + carbohydrate group.
-
Found mainly in the cell membranes of the brain and nerve cells.
-
Types:
-
Cerebrosides – contain a single sugar (glucose or galactose).
-
Gangliosides – contain complex oligosaccharides.
-
-
Functions:
-
Cell recognition and cell–cell interaction.
-
Important in nerve impulse transmission.
-
3. Lipoproteins
-
Structure: Lipid + protein complex.
-
Not structural like phospholipids, but serve as transport forms of lipids in the blood.
-
Types (based on density):
-
Chylomicrons – carry dietary triglycerides from intestine.
-
VLDL (Very Low-Density Lipoprotein) – transport triglycerides from liver.
-
LDL (Low-Density Lipoprotein) – carries cholesterol to tissues (“bad cholesterol”).
-
HDL (High-Density Lipoprotein) – carries cholesterol away to liver for excretion (“good cholesterol”).
-
-
Functions:
-
Transport of lipids (triglycerides, cholesterol) through the bloodstream.
-
-
4. Other Complex Lipids
1. Sulfolipids
-
-
Structure: Lipids containing sulfur groups, usually a sulfonic acid group (-SO₃H) attached to a sugar.
-
Example: Sulfoquinovosyl diacylglycerol (found in plants, algae, and bacteria).
-
Function:
-
Important in photosynthetic membranes (chloroplasts).
-
Found in bacterial cell walls, contributing to cell survival and virulence.
-
-
2. Aminolipids
-
-
Structure: Lipids that contain amino groups (–NH₂) in their structure.
-
Common in bacterial membranes.
-
Function:
-
Maintain membrane stability.
-
Help bacteria adapt to environmental stress (e.g., low pH).
-
Involved in interactions with host cells in pathogenic bacteria.
-
-
3. Lipo-polysaccharides (LPS)
-
-
Structure: A complex of lipid + polysaccharide.
-
Found in the outer membrane of Gram-negative bacteria.
-
Composed of:
-
Lipid A (toxic part).
-
Core polysaccharide.
-
O-antigen (antigenic determinant).
-
-
Function:
-
Provide structural integrity to bacterial outer membrane.
-
Protect bacteria from hostile environments.
-
Act as endotoxins in humans → cause fever, shock, and immune responses.
-
-
Derived Lipids or Precursor Lipids
Derived lipids are substances obtained by hydrolysis of simple and complex lipids. They are insoluble in water but soluble in organic solvents.
Examples of Derived Lipids:
-
-
Fatty acids – saturated (palmitic, stearic) & unsaturated (oleic, linoleic).
-
Glycerol – backbone of fats & oils.
-
Steroids – cholesterol, bile salts, steroid hormones (testosterone, estrogen, cortisol).
-
Terpenes & Carotenoids – vitamins A, E, K; plant pigments.
-
Eicosanoids – prostaglandins, leukotrienes, thromboxanes (act as local hormones).
-
Alcohols – long-chain alcohols (e.g., sphingosine).
-
Fat-soluble vitamins – A, D, E, K (classified as isoprenoid derivatives).
-
Miscellaneous Lipids
These are lipids that do not fit neatly into simple, complex, or derived lipids. They are diverse in structure and function.
Examples:
-
-
Carotenoids – plant pigments (β-carotene, lycopene); precursors of vitamin A.
-
Squalene – a triterpene, precursor of cholesterol and steroid hormones.
-
Eicosanoids – prostaglandins, thromboxanes, leukotrienes (local hormones).
-
Polyisoprenoids – dolichol, ubiquinone (coenzyme Q); involved in electron transport and glycoprotein synthesis.
-
Fatty acids
- Fatty acids are organic molecules that are long-chained carboxylic acids with 4-36 carbon atoms.
- Naturally occurring fatty acids are mostly unbranched, occurring in three main classes of lipids: triglycerides, phospholipids, and cholesteryl esters.
- Fatty acids are not found in the free state but remain associated with alcohol to form triglycerides.
- Fatty acids are stored as an energy reserve (fat) through an ester linkage to glycerol to form triglycerides.
Classification of Fatty Acids
Fatty acids are classified into four major classes
- Straight-chain fatty acids
- Branched-chain fatty acids
- Substituted fatty acids
- Cyclic fatty acids
1 Straight Chain Fatty
Acids Fatty acids, in which the carbons are arranged linearly, are subclassified into two classes:
-
- Saturated fatty acids
- Unsaturated fatty acids
Saturated fatty acids
There is no double bond in the hydrocarbon chain of these fatty acids. Saturated fatty acids are subclassified into two classes:
-
-
- Even carbon acids carry an even number of carbons,
-
e.g. palmitic acid and stearic acid.
-
-
- Odd carbon acids carry an odd number of carbons,
-
e.g. propionic acid.
Unsaturated fatty acids
These contain double bonds in their hydrocarbon chains.
These are subclassified according to the number of double bonds present in the structure as follows:
-
- Monoenoic or monounsaturated fatty acid
- Polyenoic or polyunsaturated fatty acid.
- Monoenoic or monounsaturated fatty acids, e.g. oleic acid.
- Polyenoic or polyunsaturated fatty acids, for example:
-
-
- Dienoic acids
- Trienoic acids,
- Tetraenoic acid,
-
2. Branched Chain Fatty Acids
These are less abundant than straight-chain acids in animals and plants, e.g.
-
- Isovaleric acid
- Isobutyric acid.
3. Substituted Fatty Acids
In substituted fatty acids, one or more hydrogen atoms have been replaced by another group, e.g.
-
- Lactic acid of blood.
- Cerebronic acid and oxynervonic acids of brain glycolipids.
- Ricinoleic acid of castor oil.
4. Cyclic Fatty Acids
Fatty acids bearing cyclic groups are present in some bacteria and seed lipids.
Functions of Fatty Acids
Fatty acids have three major physiological functions.
- They serve as building blocks of phospholipids and glycolipids. These amphipathic molecules are important components of biological membranes.
- Fatty acid derivatives serve as hormones, e.g. prostaglandins.
- Fatty acids serve as a major fuel for most cells.
Essential Fatty Acid
The fatty acids that humans require but are not synthesized in the body and hence need to be supplied in the diet are known as essential fatty acids (EFA). Humans lack the enzymes that can introduce double bonds beyond 9th Carbon.
Functions of Essential Fatty Acids
-
Cell membrane structure – maintain fluidity and permeability.
-
Precursors of eicosanoids – prostaglandins, thromboxanes, leukotrienes (regulate inflammation, blood pressure, clotting).
-
Growth & development – important for brain and vision (especially in infants).
-
Lipid transport – help in formation of lipoproteins.
-
Skin health – prevent dryness and scaling.
-
Cholesterol metabolism – lower plasma cholesterol by promoting its excretion.
Deficiency of Essential Fatty Acids
-
Poor growth & development (especially in infants/children).
-
Skin problems – dryness, scaliness, dermatitis.
-
Reproductive failure.
-
Impaired wound healing.
-
Kidney & liver problems.
-
Neurological issues – learning difficulties, vision problems (due to brain/retina effects).
-
Increased risk of infections (weakened immunity).
Significance of ω3 Fatty Acid
- Decrease the risk of cardiovascular disease.
- Appear to replace arachidonic acid in platelet membranes.
- Lower the production of Thromboxane and tendency of the platelet aggregation.
- Decrease Serum Triglycerides.
- Important for Infant Development.
- Lower the risk of various mental illnesses (Depression, ADHD)
- Lower the risk of chronic degenerative diseases such as Cancer, Rheumatoid Arthritis, and Alzheimer’s Disease.
Functions of Triglycerides
-
Energy storage – Main storage form of energy; provide ~9 kcal/g (more than carbs & proteins).
-
Energy source – Broken down to fatty acids & glycerol for ATP production.
-
Insulation – Subcutaneous fat reduces heat loss, maintaining body temperature.
-
Protection – Cushions and protects vital organs (kidneys, heart, eyes).
-
Metabolic water – Oxidation of triglycerides produces water, important in desert animals.
-
Source of glycerol & fatty acids – For synthesis of phospholipids, glycolipids, and other biomolecules.
Phospholipids
Phospholipids are complex lipids made of glycerol (or sphingosine) + 2 fatty acids + phosphate group + nitrogenous base.
-
They are amphipathic molecules → hydrophilic “head” + hydrophobic “tails”.
-
Major components of cell membranes.
Phospholipids are divided mainly into two groups based on the backbone structure:
1. Glycerophospholipids (Phosphoglycerides)
-
Backbone: Glycerol
-
General structure: Glycerol + 2 fatty acids + phosphate group + nitrogenous base
-
Major types:
-
Phosphatidylcholine (Lecithin)
-
Most abundant phospholipid in cell membranes.
-
Found in egg yolk, soybeans, brain tissue.
-
Functions: Structural role, lipid transport, lung surfactant.
-
-
Phosphatidylethanolamine (Cephalin)
-
Found in brain and nervous tissue.
-
Role in membrane structure and blood clotting.
-
-
Phosphatidylserine
-
Found in brain, inner surface of cell membranes.
-
Role in apoptosis (cell death signaling) and blood coagulation.
-
-
Phosphatidylinositol (PI)
-
Contains sugar alcohol inositol.
-
Important in signal transduction (IP₃, DAG pathway) and membrane anchoring.
-
-
Cardiolipin (Diphosphatidylglycerol)
-
Found in inner mitochondrial membrane.
-
Essential for oxidative phosphorylation and energy metabolism.
-
2. Sphingophospholipids
-
Backbone: Sphingosine (instead of glycerol).
-
General structure: Sphingosine + fatty acid + phosphate + choline/ethanolamine.
-
Major type:
Sphingomyelin
-
Found in myelin sheath of nerve fibers.
-
Role: Insulates axons, speeds up nerve impulse transmission.
Functions of Phospholipids
-
Structural role – Major components of cell membranes (lipid bilayer).
-
Membrane fluidity – Maintain the flexibility and permeability of membranes.
-
Signal transduction – Act as precursors of second messengers (e.g., IP₃, DAG).
-
Transport – Form lipoproteins that transport lipids in blood.
-
Surfactant function – Dipalmitoyl lecithin reduces surface tension in lungs (prevents alveolar collapse).
-
Nerve insulation – Sphingomyelin is important in the myelin sheath.
-
Emulsification – Helps in the digestion/absorption of dietary fats with bile.
Glycolipids
- Lipids containing carbohydrate (sugar) group + sphingosine + fatty acid.
-
Found mainly in plasma membranes, especially in nerve tissues.
-
Important for cell recognition, communication, and stability of membranes.
Classification of Glycolipids
-
Cerebrosides
-
Structure: Sphingosine + fatty acid + single sugar (glucose or galactose).
-
Example: Galactocerebroside (abundant in myelin sheath).
-
Function: Insulation of nerve fibers.
-
-
Sulfatides (sulfolipids)
-
Cerebrosides with sulfate group attached.
-
Function: Found in brain, kidney → role in cell signaling & myelin stability.
-
-
Globosides
-
Contain two or more sugars (glucose, galactose, N-acetylgalactosamine).
-
Function: Present in cell membranes, involved in cell adhesion & recognition.
-
-
Gangliosides
-
Contain complex oligosaccharides + sialic acid (NANA).
-
Highly abundant in gray matter of brain.
-
Function: Involved in nerve impulse transmission, cell–cell communication, receptor functions.
-
Functions of Glycolipids
-
Structural role – stabilize cell membranes, especially in nerve cells (myelin sheath).
-
Cell recognition & communication – act as markers for cell–cell interactions.
-
Receptors – serve as receptors for toxins, viruses, and hormones (e.g., cholera toxin binds to GM1 ganglioside).
-
Insulation of nerves – cerebrosides & sulfatides maintain myelin sheath.
-
Immune functions – glycolipids on cell surfaces act as antigens (e.g., ABO blood group antigens).
-
Signal transduction – participate in transmitting signals across membranes.
Cholesterol
Functions of Cholesterol
- It is a major structural constituent of the cell membranes and plasma lipoproteins.
- Cholesterol serves as the precursor for a variety of biologically important products, including:
- Steroid hormones: Cholesterol is the precursor of the five steroid hormones, e.g.
- Progesterones
- Glucocorticoids
- Steroid hormones: Cholesterol is the precursor of the five steroid hormones, e.g.
- Mineralocorticoids iv. Androgens (male sex hormones)
- Estrogen (female sex hormones).
- Bile acids: Bile acids, derived from cholesterol, act as a detergent in the intestine, emulsifying dietary fats to make them readily accessible to the digestive enzyme lipase.
- Vitamin D is derived from cholesterol and is essential in calcium and phosphate metabolism.
Lipoproteins
-
Lipoproteins are particles made of fats (lipids) and proteins.
-
Their job is to carry fats in the blood, because fats cannot travel alone in water (blood).
-
Structure:
-
Core (inside): contains triglycerides and cholesterol esters (water-insoluble).
-
Outer layer: has phospholipids, free cholesterol, and apolipoproteins (water-soluble).
-
They are classified based on density and function into:
1. Chylomicrons
-
Largest, lowest density lipoproteins.
-
Rich in dietary triglycerides.
-
Synthesized in intestinal mucosa after a meal.
-
Function: Transport dietary triglycerides from intestine → peripheral tissues (for energy/storage). Residue goes to liver.
2. Very Low-Density Lipoproteins (VLDL)
-
Produced by the liver.
-
Rich in endogenous triglycerides (synthesized in liver).
-
Function: Transport triglycerides from liver → peripheral tissues.
-
On metabolism, VLDL is converted → IDL → LDL.
3. Intermediate-Density Lipoproteins (IDL)
-
Transitional form between VLDL and LDL.
-
Contain triglycerides & cholesterol.
-
Function: Carry remaining triglycerides and cholesterol to tissues or liver.
4. Low-Density Lipoproteins (LDL)
-
Formed from VLDL/IDL metabolism.
-
Rich in cholesterol & cholesterol esters.
-
Function: Deliver cholesterol to peripheral tissues for membrane synthesis & hormone production.
-
Excess LDL → deposits in arteries → atherosclerosis (“bad cholesterol”).
5. High-Density Lipoproteins (HDL)
-
-
Smallest, highest density (protein-rich).
-
Synthesized in liver & intestine.
-
Function: Collect excess cholesterol from tissues & blood → transport back to liver (reverse cholesterol transport).
-
Protective role → “good cholesterol.”
-
Functions
-
Transport triglycerides (chylomicrons, VLDL).
-
Carry cholesterol to tissues (LDL = “bad cholesterol”).
-
Remove excess cholesterol back to liver (HDL = “good cholesterol”).
-
Provide energy indirectly by delivering fats to cells.
-
Help in fat absorption from intestine (chylomicrons).
