Introduction
Histopathology = study of tissues under the microscope to detect disease.
Cytopathology = study of cells under the microscope for diagnosis.
Both play a crucial role in disease detection, cancer diagnosis, prognosis, and research.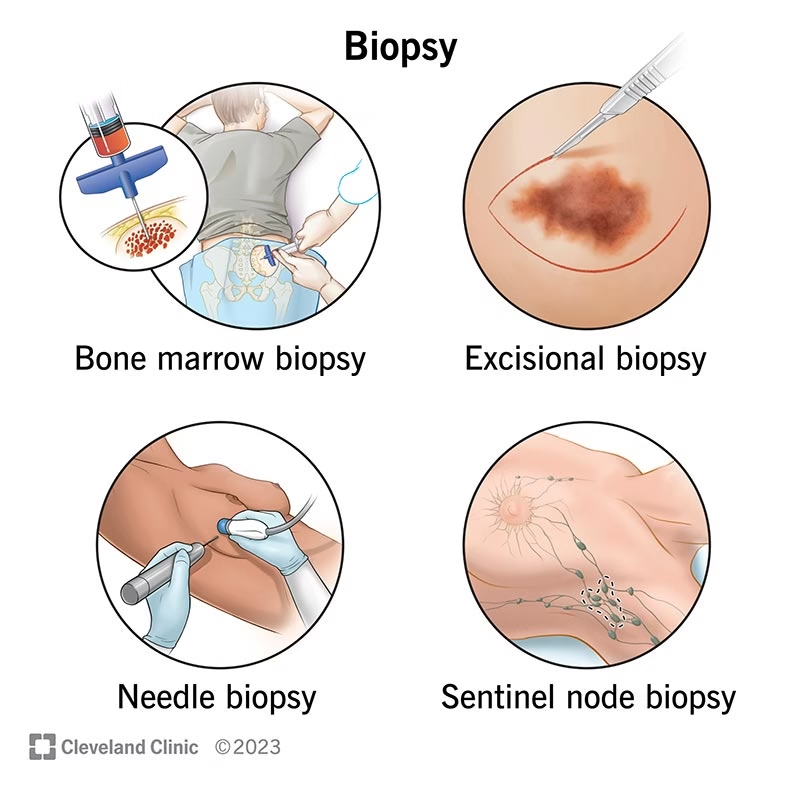
Methods of examination of tissue and cells in a histopathology lab can be divided into two broad categories:
-
Examination of Tissues (Histopathology proper)
-
Examination of Cells (Cytopathology)
Examination of Tissues
This includes methods where tissue pieces are processed and studied.
A. Biopsy
-
Definition: Removal of tissue from a living person for diagnosis.
-
Types:
-
Incisional biopsy – a small part of the lesion is removed.
-
Excisional biopsy – entire lesion removed (e.g., mole, small tumor).
-
Needle biopsy – tissue obtained by fine or core needle.
-
Endoscopic biopsy – sample taken using an endoscope (e.g., gastric, colon).
-
-
Uses: cancer diagnosis, inflammatory diseases, and infections.
B. Surgical Specimens
-
Tissues/organs removed during surgery.
-
Example: appendectomy specimen, cholecystectomy, mastectomy.
-
Process:
-
Gross examination → size, shape, color, weight, margins.
-
Sampling → representative sections cut.
-
Microscopy after processing and staining.
-
C. Autopsy
-
Examination of tissues after death.
-
Types:
-
Clinical autopsy (cause of death).
-
Medico-legal autopsy (suspicious deaths).
-
-
Provides information on disease progression, response to treatment, genetic disorders.
D. Tissue Processing
Before microscopic examination, tissue undergoes stepwise preparation:
-
Fixation
-
Preserves tissue, prevents decomposition.
-
Common fixative: 10% formalin.
-
Other fixatives: Bouin’s, Carnoy’s, glutaraldehyde (for EM).
-
-
Dehydration
-
Tissue passed through increasing grades of alcohol to remove water.
-
-
Clearing
-
Alcohol replaced with xylene or chloroform.
-
-
Embedding
-
Tissue infiltrated with molten paraffin wax → solid block formed.
-
-
Sectioning
-
Thin slices (3–5 µm) cut by microtome.
-
-
Staining
-
Routine stain: Hematoxylin & Eosin (H&E).
-
Hematoxylin → stains nuclei blue/purple.
-
Eosin → stains cytoplasm pink.
-
-
E. Special Stains
Used when H&E is not sufficient.
-
Periodic Acid–Schiff (PAS) → glycogen, mucin.
-
Ziehl–Neelsen stain → Mycobacterium tuberculosis.
-
Silver stains → fungi, reticulin fibers.
-
Congo Red → amyloid (apple-green birefringence in polarized light).
-
Masson’s Trichrome → collagen.
F. Immunohistochemistry
-
Uses antigen–antibody reactions to detect proteins in tissue.
-
Example:
-
ER/PR, HER2 in breast cancer (for targeted therapy).
-
Ki-67 (proliferation marker).
-
-
Advantage: provides diagnosis, prognosis, and therapeutic guidance.
G. Frozen Section
-
Tissue rapidly frozen in cryostat, sectioned, stained, and examined.
-
Provides quick diagnosis during surgery.
-
Example: checking tumor margins in breast cancer surgery.
H. Electron Microscopy
-
Provides ultrastructural details at very high magnification.
-
Used in:
-
Renal biopsies (glomerular diseases).
-
Muscle biopsies.
-
Viral identification.
-
Examination of Cells
Here, individual cells or clusters are studied without intact tissue architecture.
A. Exfoliative Cytology
-
Cells naturally shed or mechanically scraped.
-
Most famous: Pap smear (cervical cancer screening).
-
Also: sputum cytology, urine cytology.
B. Fine Needle Aspiration Cytology
-
Technique: Fine needle (22–25G) inserted into lump/swelling, cells aspirated, smeared on slide, stained.
-
Stains: May–Grünwald Giemsa (MGG), Papanicolaou.
-
Advantages: Quick, cheap, outpatient procedure, minimal risk.
-
Uses: breast lump, thyroid nodule, lymph node, salivary gland, soft tissue swelling.
C. Body Fluids Cytology
-
Examining cells in:
-
Pleural fluid
-
Peritoneal/ascitic fluid
-
Cerebrospinal fluid (CSF)
-
Synovial fluid
-
-
Detects infections (TB, bacteria), malignancy (metastatic cells).
D. Imprint Cytology (Touch Prep)
-
Fresh tissue touched on slide → cell imprint → stained and studied.
-
Rapid diagnosis in breast, thyroid, lymph nodes.
E. Cytochemistry
-
Uses chemical stains to highlight cell contents.
-
Example:
-
Sudan Black → lipids (used in leukemias).
-
PAS → glycogen.
-
Peroxidase stain → myeloid cells.
-
F. Flow Cytometry
-
Cells in suspension are passed through laser beam → fluorescent antibodies used to detect cell markers.
-
Provides immunophenotyping.
-
Uses:
-
Leukemia & lymphoma classification.
-
Detection of minimal residual disease.
-
Comparison Table: Tissue vs. Cell Examination
| Feature | Tissue (Histopathology) | Cells (Cytopathology) |
|---|---|---|
| Sample | Biopsy, surgical specimen | FNAC, Pap smear, body fluids |
| Architecture seen? | Yes (tissue pattern preserved) | No (only single cells) |
| Processing time | Long (fixation, embedding, sectioning) | Short (smear, stain, examine) |
| Common stains | H&E, special stains, IHC | Pap, MGG, cytochemistry |
| Use | Tumor typing, staging, prognosis | Screening, rapid diagnosis |
| Example | Breast carcinoma biopsy | Breast lump FNAC |