Introduction
-
Quantitative assays of coagulation factors play a vital role in the diagnosis, classification, and management of bleeding disorders.
-
These assays are designed to measure the concentration or functional activity of specific clotting factors present in blood plasma.
-
Deficiency or dysfunction of coagulation factors can lead to inherited or acquired haemostatic disorders, resulting in abnormal bleeding tendencies.
-
Accurate quantification of individual clotting factors helps in identifying the exact factor involved in a bleeding disorder.
-
Such assays are essential for confirming diagnoses like haemophilia, von Willebrand disease, and other rare coagulation factor deficiencies.
-
Quantitative coagulation assays also assist in monitoring disease severity and guiding appropriate replacement therapy.
-
They are routinely used to evaluate patient response to treatment and to adjust dosage of clotting factor concentrates.
-
In addition, these assays are important in preoperative assessment to prevent bleeding complications during surgical procedures.
-
Various laboratory methods are available for quantitative estimation of coagulation factors, each based on different principles and clinical applications.
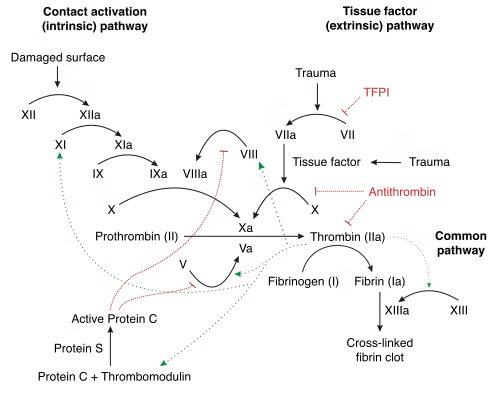
Quantitative assays of coagulation
Factor VIII Assay
Principle
-
The Factor VIII assay is most commonly performed by the one-stage clotting assay method.
-
The principle is based on the ability of patient plasma to correct the prolonged Activated Partial Thromboplastin Time (APTT) of Factor VIII–deficient plasma.
-
When patient plasma containing Factor VIII is mixed with Factor VIII–deficient plasma, the clotting time shortens in proportion to the Factor VIII activity present.
-
The clotting time obtained is compared with a calibration curve prepared using normal reference plasma of known Factor VIII activity.
-
The result is expressed as percentage activity (%) or IU/dL of Factor VIII.
Sample
-
Venous blood collected in 3.2% sodium citrate anticoagulant.
-
Blood to anticoagulant ratio should be 9 : 1.
-
Platelet-poor plasma (PPP) is prepared by centrifugation at 1500–2000 g for 15 minutes.
-
Plasma should be tested immediately or stored at –20°C to –70°C if delayed.
Requirements
-
Patient citrated plasma
-
Normal pooled plasma (reference plasma)
-
Factor VIII–deficient plasma
-
APTT reagent
-
Calcium chloride (0.025 M)
-
Coagulometer or water bath at 37°C
-
Test tubes and pipettes
-
Stopwatch (if manual method is used)
Procedure
-
Prepare serial dilutions of normal pooled plasma to construct a standard calibration curve.
-
Dilute patient plasma similarly as per laboratory protocol.
-
Mix diluted patient plasma with Factor VIII–deficient plasma in a test tube.
-
Add APTT reagent and incubate the mixture at 37°C for the specified time.
-
Add pre-warmed calcium chloride to initiate clotting.
-
Record the clotting time using a coagulometer or stopwatch.
-
Repeat the same steps for standard plasma dilutions.
-
Plot clotting times of standards to generate a calibration curve.
-
Determine the Factor VIII activity of patient plasma by comparing clotting time with the standard curve.
Results
-
Results are expressed as Factor VIII activity (%) or IU/dL.
-
Normal range: approximately 50–150%.
-
Mild haemophilia A: 5–40%
-
Moderate haemophilia A: 1–5%
-
Severe haemophilia A: <1%
-
Reduced levels indicate Factor VIII deficiency or dysfunction.
-
Normal or elevated levels may be seen in stress, pregnancy, inflammation, or acute phase reactions.
Factor IX Assay
Principle
-
The Factor IX assay is a one-stage clotting assay based on the Activated Partial Thromboplastin Time (APTT).
-
The test measures the ability of patient plasma to correct the prolonged APTT of Factor IX–deficient plasma.
-
When patient plasma containing Factor IX is mixed with Factor IX–deficient plasma, the clotting time shortens in direct proportion to the Factor IX activity present.
-
The clotting time is compared with a standard calibration curve prepared using normal pooled plasma of known Factor IX activity.
-
Results are expressed as percentage activity (%) or IU/dL.
Sample
-
Venous blood collected in 3.2% sodium citrate anticoagulant.
-
Blood to anticoagulant ratio should be 9 : 1.
-
Platelet-poor plasma is prepared by centrifugation at 1500–2000 g for 15 minutes.
-
Plasma should be tested immediately or stored frozen at –20°C to –70°C if testing is delayed.
Requirements
-
Patient citrated plasma
-
Normal pooled plasma (reference plasma)
-
Factor IX–deficient plasma
-
APTT reagent
-
Calcium chloride (0.025 M)
-
Coagulometer or water bath maintained at 37°C
-
Test tubes, pipettes, and timer/stopwatch
Procedure
-
Prepare serial dilutions of normal pooled plasma to generate a standard calibration curve.
-
Dilute patient plasma according to laboratory protocol.
-
Mix diluted patient plasma with Factor IX–deficient plasma.
-
Add APTT reagent and incubate the mixture at 37°C for the specified time.
-
Add pre-warmed calcium chloride to initiate clot formation.
-
Measure and record the clotting time.
-
Perform the same steps for standard plasma dilutions.
-
Plot clotting times of standards to construct a calibration curve.
-
Determine Factor IX activity in patient plasma by comparing clotting time with the standard curve.
Results
-
Results are reported as Factor IX activity (%) or IU/dL.
-
Normal range: approximately 50–150%.
-
Mild haemophilia B: 5–40%
-
Moderate haemophilia B: 1–5%
-
Severe haemophilia B: <1%
-
Decreased levels indicate Factor IX deficiency, commonly seen in haemophilia B or acquired coagulation disorders.
-
Normal or increased levels may be observed in acute phase reactions or inflammatory states.
Factor XIII Assay
Principle
-
Factor XIII assay is used to assess the activity or presence of Factor XIII (fibrin-stabilizing factor), which plays a crucial role in the final stage of coagulation.
-
Unlike other coagulation factors, Factor XIII does not affect PT or APTT, so routine coagulation tests remain normal.
-
The assay principle is based on the ability of Factor XIII to stabilize fibrin clots by cross-linking fibrin monomers.
-
In the commonly used clot solubility test, a fibrin clot formed in the presence of calcium is exposed to 5 M urea or 1% monochloroacetic acid.
-
If Factor XIII is deficient, the clot dissolves due to lack of cross-linking; a stable clot indicates normal Factor XIII activity.
Sample
-
Venous blood collected in 3.2% sodium citrate anticoagulant.
-
Blood to anticoagulant ratio: 9 : 1.
-
Platelet-poor plasma prepared by centrifugation at 1500–2000 g for 15 minutes.
-
Plasma should be tested fresh or stored frozen at –20°C if delayed.
Requirements
-
Patient citrated plasma
-
Normal pooled plasma (control)
-
Calcium chloride solution
-
Thrombin solution
-
5 M urea solution or 1% monochloroacetic acid
-
Test tubes and pipettes
-
Water bath/incubator at 37°C
-
Timer/stopwatch
Procedure (Clot Solubility Method)
-
Take patient plasma in a clean test tube.
-
Add thrombin and calcium chloride to form a fibrin clot.
-
Incubate the tube at 37°C until a firm clot is formed.
-
Carefully add 5 M urea (or 1% monochloroacetic acid) without disturbing the clot.
-
Incubate at room temperature or 37°C for 24 hours.
-
Observe the clot for dissolution at regular intervals.
-
Perform the same procedure with normal pooled plasma as control.
Results
-
Normal Factor XIII activity: Clot remains intact and stable for 24 hours.
-
Factor XIII deficiency: Clot dissolves partially or completely within 24 hours.
-
This test is qualitative or semi-quantitative and detects only severe Factor XIII deficiency (<1–5%).
-
Normal result does not exclude mild or moderate deficiency, for which immunological or chromogenic assays are required.
Fibrinogen Assay
Principle
-
The fibrinogen assay is a quantitative test used to measure the concentration of plasma fibrinogen (Factor I).
-
The most commonly used method is the Clauss method, which is a functional clot-based assay.
-
In this method, a high concentration of thrombin is added to diluted patient plasma, converting fibrinogen to fibrin.
-
The time taken for clot formation is inversely proportional to the fibrinogen concentration in the plasma.
-
Clotting time is compared with a calibration curve prepared using reference plasma of known fibrinogen concentration.
-
Results are expressed in mg/dL or g/L.
Sample
-
Venous blood collected in 3.2% sodium citrate anticoagulant.
-
Blood to anticoagulant ratio: 9 : 1.
-
Platelet-poor plasma prepared by centrifugation at 1500–2000 g for 15 minutes.
-
Plasma should be tested promptly or stored frozen at –20°C to –70°C if delayed.
Requirements
-
Patient citrated plasma
-
Normal pooled plasma or fibrinogen calibrator
-
Thrombin reagent (high concentration)
-
Buffer/diluent
-
Coagulometer or water bath at 37°C
-
Test tubes, pipettes, and timer
Procedure (Clauss Method)
-
Dilute patient plasma with buffer as per kit or laboratory protocol.
-
Prepare fibrinogen standards from reference plasma to generate a calibration curve.
-
Incubate diluted plasma at 37°C.
-
Add a fixed volume of thrombin reagent to the plasma.
-
Measure the clotting time using a coagulometer or stopwatch.
-
Perform the same steps for calibration standards.
-
Plot clotting time against fibrinogen concentration to prepare a standard curve.
-
Determine fibrinogen concentration of the patient sample from the curve.
Results
-
Results are reported as fibrinogen concentration (mg/dL or g/L).
-
Normal range: approximately 200–400 mg/dL (2–4 g/L).
-
Decreased levels are seen in:
-
Disseminated intravascular coagulation (DIC)
-
Severe liver disease
-
Congenital afibrinogenemia or hypofibrinogenemia
-
Massive hemorrhage
-
-
Increased levels are seen in:
-
Acute phase reactions
-
Pregnancy
-
Inflammatory conditions and infections
-
Antithrombin III Assay
Principle
-
The Antithrombin III (AT III) assay is a quantitative test used to measure the functional activity or antigenic level of antithrombin, a major natural anticoagulant present in plasma.
-
Antithrombin inhibits several activated coagulation factors, mainly thrombin (Factor IIa) and Factor Xa, thereby regulating coagulation.
-
The most commonly used method is the chromogenic functional assay.
-
In this method, patient plasma is incubated with an excess of thrombin or Factor Xa in the presence of heparin, which accelerates antithrombin activity.
-
Residual (uninhibited) enzyme cleaves a chromogenic substrate, releasing a colored compound.
-
The color intensity is inversely proportional to the antithrombin activity in the sample.
Sample
-
Venous blood collected in 3.2% sodium citrate anticoagulant.
-
Blood to anticoagulant ratio: 9 : 1.
-
Platelet-poor plasma prepared by centrifugation at 1500–2000 g for 15 minutes.
-
Plasma should be tested immediately or stored frozen at –20°C to –70°C if delayed.
Requirements
-
Patient citrated plasma
-
Antithrombin calibrator or normal pooled plasma
-
Thrombin or Factor Xa reagent
-
Heparin reagent
-
Chromogenic substrate
-
Buffer solution
-
Spectrophotometer or automated coagulation analyzer
-
Test tubes, pipettes, incubator at 37°C
Procedure (Chromogenic Method)
-
Dilute patient plasma according to kit or laboratory protocol.
-
Incubate plasma with excess thrombin or Factor Xa and heparin at 37°C.
-
Allow sufficient time for antithrombin to inactivate the enzyme.
-
Add chromogenic substrate to the reaction mixture.
-
Measure the absorbance of the released colored product using a spectrophotometer.
-
Perform the same steps for calibrators and controls.
-
Prepare a calibration curve from standard samples.
-
Determine antithrombin activity of patient plasma by comparing absorbance values.
Results
-
Results are expressed as Antithrombin activity (%) or IU/mL.
-
Normal range: approximately 80–120%.
-
Decreased levels are seen in:
-
Inherited antithrombin deficiency (Type I and Type II)
-
Disseminated intravascular coagulation (DIC)
-
Liver disease
-
Nephrotic syndrome
-
Heparin therapy (consumptive loss)
-
-
Increased levels are rare and usually of no clinical significance.
Special Considerations
-
Calibration and Standards:
-
Each coagulation assay must be calibrated using standard preparations with known factor concentrations.
-
Calibration curves are essential for accurate quantification of coagulation factors.
-
Recalibration is required when there is a change in reagent lot or instrument.
-
-
Quality Control:
-
Routine use of normal and abnormal quality control samples is necessary.
-
Quality control helps in assessing accuracy, precision, and reliability of test results.
-
Regular internal and external quality assurance ensures consistent assay performance.
-
-
Clinical Correlation:
-
Test results should always be correlated with clinical history and symptoms.
-
Interpretation must consider other laboratory parameters such as PT, APTT, and platelet count.
-
Proper clinical correlation is crucial for accurate diagnosis and effective patient management.
-
MCQs
1. Quantitative coagulation assays are mainly used to measure:
A. Platelet count
B. Bleeding time
C. Specific clotting factor levels
D. Hemoglobin concentration
Answer: C
2. Factor VIII deficiency is classically associated with:
A. Hemophilia B
B. Hemophilia A
C. von Willebrand disease only
D. DIC
Answer: B
3. The most common method for Factor VIII assay is:
A. PT-based method
B. Thrombin time
C. One-stage clotting assay
D. ELISA
Answer: C
4. Factor VIII assay is based on correction of prolonged:
A. PT
B. Bleeding time
C. APTT
D. Thrombin time
Answer: C
5. Factor VIII–deficient plasma lacks:
A. Factor IX
B. Factor XI
C. Factor VIII
D. Factor XIII
Answer: C
6. Normal Factor VIII activity range is approximately:
A. 10–40%
B. 20–60%
C. 50–150%
D. 150–300%
Answer: C
7. Severe hemophilia A shows Factor VIII levels:
A. <1%
B. 5–10%
C. 10–30%
D. >50%
Answer: A
8. Factor IX deficiency causes:
A. Hemophilia A
B. Hemophilia B
C. DIC
D. von Willebrand disease
Answer: B
9. Factor IX assay also uses which test principle?
A. PT correction
B. APTT correction
C. Thrombin time
D. Bleeding time
Answer: B
10. Normal Factor IX activity range is:
A. 5–40%
B. 10–50%
C. 50–150%
D. 200–400%
Answer: C
11. Which coagulation factor does NOT affect PT or APTT?
A. Factor VIII
B. Factor IX
C. Factor XI
D. Factor XIII
Answer: D
12. Factor XIII is also known as:
A. Prothrombin
B. Christmas factor
C. Fibrin-stabilizing factor
D. Antihemophilic factor
Answer: C
13. Routine coagulation tests in Factor XIII deficiency are:
A. Prolonged
B. Normal
C. Increased
D. Variable
Answer: B
14. Factor XIII assay is commonly performed by:
A. Clauss method
B. ELISA
C. Clot solubility test
D. PT-derived method
Answer: C
15. Reagent used in clot solubility test is:
A. Sodium citrate
B. Calcium chloride
C. 5 M urea
D. EDTA
Answer: C
16. Dissolution of clot within 24 hours indicates:
A. Normal Factor XIII
B. Increased fibrinogen
C. Factor XIII deficiency
D. Heparin excess
Answer: C
17. Fibrinogen is also known as:
A. Factor II
B. Factor I
C. Factor V
D. Factor XIII
Answer: B
18. Most commonly used fibrinogen assay method is:
A. ELISA
B. Immunoturbidimetry
C. Clauss method
D. APTT method
Answer: C
19. Clauss method is a:
A. Antigenic assay
B. Functional assay
C. Platelet assay
D. Immunological assay
Answer: B
20. In Clauss method, clotting time is:
A. Directly proportional to fibrinogen
B. Independent of fibrinogen
C. Inversely proportional to fibrinogen
D. Equal to PT
Answer: C
21. Normal plasma fibrinogen range is:
A. 50–100 mg/dL
B. 100–200 mg/dL
C. 200–400 mg/dL
D. 500–700 mg/dL
Answer: C
22. Decreased fibrinogen levels are seen in:
A. Pregnancy
B. Inflammation
C. DIC
D. Acute phase response
Answer: C
23. Antithrombin III is a:
A. Procoagulant
B. Platelet factor
C. Natural anticoagulant
D. Vitamin K–dependent factor
Answer: C
24. Antithrombin mainly inhibits:
A. Factor VIII
B. Factor V
C. Thrombin and Factor Xa
D. Factor XIII
Answer: C
25. Most common AT III assay method is:
A. One-stage clotting
B. Immunodiffusion
C. Chromogenic assay
D. PT method
Answer: C
26. In chromogenic AT III assay, color intensity is:
A. Directly proportional to AT III
B. Inversely proportional to AT III
C. Unrelated to AT III
D. Equal in all samples
Answer: B
27. Normal Antithrombin III activity range is:
A. 20–50%
B. 50–70%
C. 80–120%
D. 150–200%
Answer: C
28. Inherited AT III deficiency predisposes to:
A. Bleeding
B. Thrombosis
C. Anemia
D. Leukemia
Answer: B
29. Antithrombin levels are reduced in:
A. Pregnancy
B. Nephrotic syndrome
C. Polycythemia
D. Iron deficiency
Answer: B
30. Anticoagulant used for coagulation assays is:
A. EDTA
B. Heparin
C. Sodium citrate
D. Oxalate
Answer: C
31. Recommended blood-to-anticoagulant ratio is:
A. 1:1
B. 5:1
C. 9:1
D. 10:1
Answer: C
32. Platelet-poor plasma is prepared by:
A. Slow centrifugation
B. Filtration
C. High-speed centrifugation
D. Freezing
Answer: C
33. Calibration in coagulation assays is done using:
A. Patient plasma
B. Saline
C. Standard plasma
D. Distilled water
Answer: C
34. Quality control samples are used to assess:
A. Patient diagnosis
B. Test accuracy and precision
C. Disease severity
D. Blood grouping
Answer: B
35. Which factor assay uses APTT correction?
A. Factor XIII
B. Factor I
C. Factor VIII
D. Antithrombin
Answer: C
36. Factor IX assay principle is similar to:
A. PT
B. TT
C. Factor VIII assay
D. Factor XIII assay
Answer: C
37. Delayed bleeding with normal PT and APTT suggests:
A. Hemophilia A
B. Hemophilia B
C. Factor XIII deficiency
D. DIC
Answer: C
38. Which assay detects severe Factor XIII deficiency only?
A. Chromogenic assay
B. Immunoassay
C. Clot solubility test
D. Clauss method
Answer: C
39. Elevated fibrinogen levels are seen in:
A. Liver failure
B. DIC
C. Acute inflammation
D. Afibrinogenemia
Answer: C
40. Heparin enhances activity of:
A. Protein C
B. Protein S
C. Antithrombin III
D. Factor XIII
Answer: C
41. Which factor is an acute-phase reactant?
A. Factor VIII
B. Factor IX
C. Factor XIII
D. Antithrombin
Answer: A
42. Factor assays results are usually expressed in:
A. mg/dL only
B. Seconds
C. Percentage or IU/dL
D. mmol/L
Answer: C
43. Improper sample collection may cause:
A. False results
B. Increased accuracy
C. Normalization
D. Calibration
Answer: A
44. Which test monitors fibrinogen function?
A. PT
B. Clauss method
C. Bleeding time
D. Platelet count
Answer: B
45. Antithrombin assay is important in patients with:
A. Recurrent bleeding
B. Recurrent thrombosis
C. Leukopenia
D. Anemia
Answer: B
46. Factor VIII inhibitor is suspected when:
A. PT is prolonged
B. APTT corrects fully
C. APTT fails to correct
D. TT is prolonged
Answer: C
47. Storage temperature for plasma if delayed testing is:
A. Room temperature
B. 4°C
C. –20°C or below
D. 60°C
Answer: C
48. Quality control should be run:
A. Once a year
B. Only when error occurs
C. Regularly with assays
D. Never
Answer: C
49. Clinical correlation means:
A. Reporting results only
B. Ignoring symptoms
C. Interpreting results with patient findings
D. Repeating test always
Answer: C
50. Quantitative coagulation assays are MOST useful for:
A. Blood grouping
B. Screening donors
C. Diagnosing specific factor deficiencies
D. Hemoglobin estimation
Answer: C