
- Ultramicrotomy is a precision technique used in electron microscopy to prepare ultra-thin slices (sections) of specimens.
- These thin sections are essential for techniques like transmission electron microscopy (TEM), where the electron beam needs to penetrate the specimen to create high-resolution images.
- Ultramicrotomy is also used in scanning electron microscopy (SEM) for certain applications, such as serial block-face imaging.
- Ultramicrotomy is a specialized technique that involves cutting extremely thin sections from biological or material specimens.
- The primary objective is to achieve sections thin enough (typically between 50-100 nm) for transmission electron microscopy (TEM) or other imaging applications that require electron beam penetration.
Applications of Ultramicrotomy
- Biological TEM: Allows imaging of cell organelles, membranes, and fine structural details of tissues and cells.
- Materials Science: Used to prepare ultra-thin sections of metals, polymers, ceramics, and other composite materials.
- Serial Block-Face Imaging: Sections are sequentially removed and imaged to reconstruct three-dimensional models, especially in neural mapping.
Components and Structure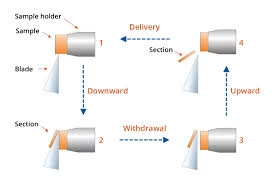
- Specimen Block and Holder
- Embedding in Resin: Specimens are typically embedded in an epoxy or acrylic resin, providing a stable medium for sectioning.
- Trimming and Shaping the Block: The embedded specimen is trimmed to create a small, smooth cutting face to ensure clean sections.
- Specimen Holder: This securely holds the resin block in position, adjusted for sectioning under precise alignment.
- The Knife
Ultramicrotomes use different types of knives based on the specimen type, cutting precision, and cost considerations:
- Diamond Knives: Extremely sharp, durable, and produce high-quality, consistent sections. Diamond knives are highly resistant to wear and are preferred for biological and harder material samples.
- Glass Knives: More affordable but less durable than diamond knives. Typically used for soft biological specimens and for producing semi-thin sections (0.5–2 µm) before switching to diamond for ultrathin cuts.
- Vibrating and Cryo-Ultramicrotomes
Specialized ultramicrotomes have been developed for different types of sectioning tasks:
- Vibrating Ultramicrotome: Used to cut thicker sections and typically used for light microscopy, although some adaptations allow thinner sections for EM.
- Cryo-Ultramicrotome: Operates at cryogenic temperatures, allowing for sectioning of frozen-hydrated specimens without the need for dehydration or embedding. This is particularly useful for highly hydrated samples or sensitive to heat, such as proteins or lipid-rich tissues.
- Water Trough
The water trough sits directly below the knife and serves two main purposes:
- Collecting Sections: As sections are cut, they float on the water surface, minimizing damage and making them easier to retrieve.
- Reducing Artifacts: Water provides a stable medium that helps prevent the formation of compression or wrinkles in the section as it is being cut.
- Thickness Control Mechanism
This component allows precise control of section thickness, usually adjustable down to increments of 10 nm. Ultramicrotomes typically have digital thickness settings, providing accuracy and repeatability.
Ultramicrotomy Process
- Specimen Fixation:
- Fixation stabilizes cellular structures, typically using glutaraldehyde (for protein cross-linking) followed by osmium tetroxide (for lipid stabilization).
- This process is especially critical for biological samples to preserve the fine structure of organelles and membranes.
- Dehydration:
- Specimens are gradually dehydrated in graded ethanol or acetone solutions to remove water, preventing structural collapse or damage during embedding.
- In cryo-ultramicrotomy, dehydration may be skipped if the sample is frozen.
- Embedding:
- The specimen is embedded in a resin (such as epoxy or acrylic) and polymerized, creating a stable block around the sample. This block is attached to a metal holder, ensuring rigidity and stability during sectioning.
- Block Trimming:
- The resin block is trimmed to expose a small area of the specimen for sectioning, typically in a trapezoidal or square shape to allow controlled, uniform cuts.
- Ultrathin Sectioning:
- The specimen holder and block are mounted onto the ultramicrotome, and the diamond or glass knife is precisely aligned.
- The knife edge slices through the specimen, producing thickness sections between 50–100 nm.
- Section thickness is controlled via adjustments in the microtome settings, and precise thickness is crucial as even slight variations can affect imaging quality in TEM.
- Section Retrieval:
- The sections float on the water surface of the trough. They are then collected using a small loop or transferred directly to a grid using forceps. Proper technique is critical here to avoid folding or wrinkling.
- Staining:
- After retrieval, sections are often stained with heavy metals (e.g., uranyl acetate and lead citrate) to enhance contrast by increasing electron density. This step is essential for distinguishing fine structural details in biological samples.
- Drying and Mounting:
- Sections on grids are carefully dried and may be examined under the electron microscope or subjected to further processing if required.
Challenges and Artifacts in Ultramicrotomy
Common Issues
- Chatter Marks: Ripple-like artifacts caused by mechanical vibrations, often due to an improperly aligned knife or inconsistencies in specimen hardness.
- Compression and Stretching: Results in distorted images, often due to the cutting angle, resin quality, or knife condition.
- Knife Marks: Fine scratches or marks from dull or damaged knives can appear on the sections.
Minimizing Artifacts
- Knife Condition: Regular cleaning, sharpening, or replacement of the knife is essential to prevent cutting artifacts.
- Embedding Quality: Using high-quality, properly polymerized resin minimizes sectioning artifacts.
- Proper Alignment: Precise alignment of the specimen block with the knife edge ensures even sectioning.
Types of Sections and Their Uses
- Ultrathin Sections: Typically 50–100 nm thick, suitable for TEM imaging and analysis of cellular structures, organelles, and nano-scale materials.
- Semi-thin Sections: Approximately 500 nm – 2 µm thick, used for initial orientation and light microscopy.
- Cryo-Sections: Produced at cryogenic temperatures for specimens that need to maintain their hydrated state, common in structural biology and cryo-electron tomography.
Advanced Techniques Related to Ultramicrotomy
Serial Block-Face Scanning Electron Microscopy (SBF-SEM)
- Method: Successive ultrathin sections are cut from the block, and SEM images the exposed face.
- Application: Creates a 3D dataset for reconstructing the architecture of complex biological tissues, such as brain tissue in neural circuit mapping.
Array Tomography
- Method: Involves serial sectioning and collection of hundreds or thousands of thin sections on a single substrate for light or electron microscopy.
- Application: Often used for studying protein localization and cell morphology at high resolution.
Cryo-Ultramicrotomy
- Method: Ultrathin sections of frozen, hydrated samples are cut at temperatures below -120°C, typically without embedding.
- Application: Used to maintain native hydration of biological specimens, enabling imaging of structures in their close-to-native state, especially in cryo-TEM.
Advantages of Ultramicrotomy
- High-Resolution Imaging: Enables imaging of ultra-thin sections at resolutions suitable for visualizing cellular structures down to the molecular level.
- Versatile Application: Applicable in biological and materials science for examining internal structure, morphology, and composition.
- 3D Reconstruction: Allows for serial sectioning, facilitating three-dimensional reconstructions and spatial analysis of complex structures.
Limitations of Ultramicrotomy
- Specimen Preparation Time: Embedding and sectioning are labor-intensive and require skilled handling to avoid damage.
- Fragile Sections: Ultra-thin sections are delicate and prone to folding, wrinkling, or tearing, requiring careful handling and precise technique.
- Potential Artifacts: Cutting artifacts, compression, and deformation can affect the interpretability of images.
Best Practices for Ultramicrotomy
- Use a Diamond Knife for Consistency: Diamond knives produce high-quality cuts and last longer than glass knives, especially for hard or mixed composition samples.
- Ensure Proper Resin Polymerization: Incomplete polymerization can lead to soft spots, causing inconsistency in sectioning.
- Optimize Cutting Speed and Angle: Adjusting cutting speed and knife angle can reduce the likelihood of chatter or compression.
- Cryo-Ultramicrotomy for Sensitive Samples: Cryo-ultramicrotomy is ideal for samples requiring a hydrated state or minimal structural alteration.
- Control Environment: Minimize vibrations and maintain a clean environment to prevent contamination and reduce cutting artifacts.